COMPLETE
Clip, Removable (skin)
RICHARD-ALLAN MEDICAL IND., INC.
The following data is part of a premarket notification filed by Richard-allan Medical Ind., Inc. with the FDA for Complete.
Pre-market Notification Details
| Device ID | K822145 |
| 510k Number | K822145 |
| Device Name: | COMPLETE |
| Classification | Clip, Removable (skin) |
| Applicant | RICHARD-ALLAN MEDICAL IND., INC. 803 N. Front St. Suite 3 Mchenry, IL 60050 |
| Product Code | FZQ |
| CFR Regulation Number | 878.4320 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 1982-07-21 |
| Decision Date | 1982-10-22 |
Trademark Results [COMPLETE]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 COMPLETE 97354349 not registered Live/Pending |
AbbVie Biotechnology Ltd 2022-04-08 |
 COMPLETE 88371368 not registered Live/Pending |
Walmart Apollo, LLC 2019-04-04 |
 COMPLETE 88368566 not registered Live/Pending |
Gloria S.A. 2019-04-02 |
 COMPLETE 88355297 5873262 Live/Registered |
Complete Mobile Canning, LLC 2019-03-25 |
 COMPLETE 87165255 5649753 Live/Registered |
NDL Industries Inc. 2016-09-08 |
 COMPLETE 87043367 5244100 Live/Registered |
Masterchem Industries LLC 2016-05-19 |
 COMPLETE 87002181 5243951 Live/Registered |
PBM Products, L.L.C. 2016-04-15 |
 COMPLETE 86447790 not registered Dead/Abandoned |
Engage Agro USA LLC 2014-11-07 |
 COMPLETE 86302475 4745072 Live/Registered |
Schultz, Alexander 2014-06-06 |
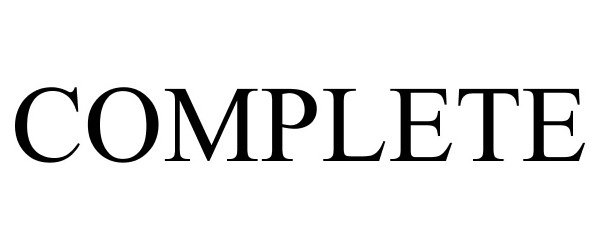 COMPLETE 86179041 4750497 Live/Registered |
Elswood Investment Corporation 2014-01-29 |
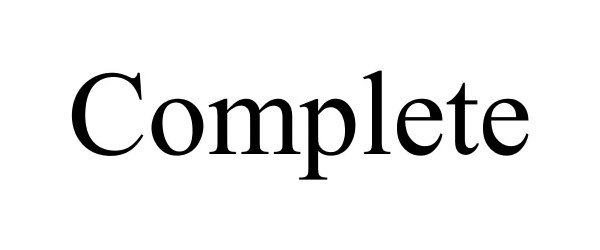 COMPLETE 86177409 4576007 Live/Registered |
WINFIELD SOLUTIONS, LLC 2014-01-28 |
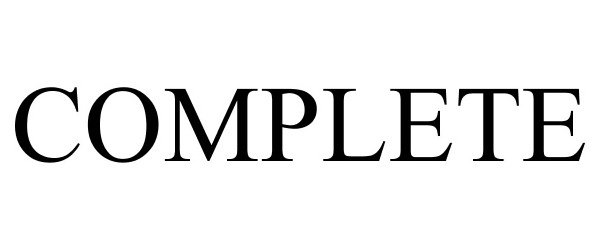 COMPLETE 85804173 4368576 Live/Registered |
PROSIGHT GLOBAL, INC. 2012-12-17 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.