MARK V
Injector And Syringe, Angiographic
MEDRAD, INC.
The following data is part of a premarket notification filed by Medrad, Inc. with the FDA for Mark V.
Pre-market Notification Details
| Device ID | K822536 |
| 510k Number | K822536 |
| Device Name: | MARK V |
| Classification | Injector And Syringe, Angiographic |
| Applicant | MEDRAD, INC. 4221 Richmond Rd., N.W. Walker, MI 49534 |
| Product Code | DXT |
| CFR Regulation Number | 870.1650 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 1982-08-24 |
| Decision Date | 1982-09-28 |
NIH GUDID Devices
| Device Identifier | submissionNumber | Supplement |
|---|---|---|
| 40616258011002 | K822536 | 000 |
| 40616258009931 | K822536 | 000 |
| 40616258024347 | K822536 | 000 |
| 40616258024330 | K822536 | 000 |
Trademark Results [MARK V]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 MARK V 90739456 not registered Live/Pending |
SAF-HOLLAND, Inc. 2021-05-27 |
 MARK V 88614601 not registered Live/Pending |
Bestway Oilfield, Inc. 2019-09-12 |
 MARK V 88311315 not registered Live/Pending |
Distillers Way, LLC 2019-02-21 |
 MARK V 87926229 5654577 Live/Registered |
Graco Minnesota Inc. 2018-05-17 |
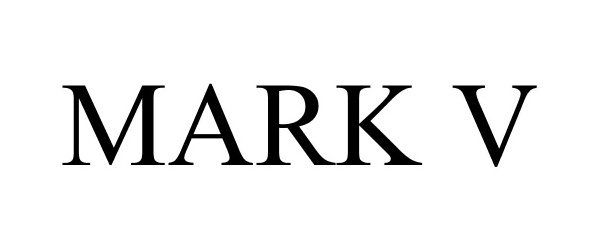 MARK V 86692373 not registered Dead/Abandoned |
VT Halter Marine, Inc. 2015-07-14 |
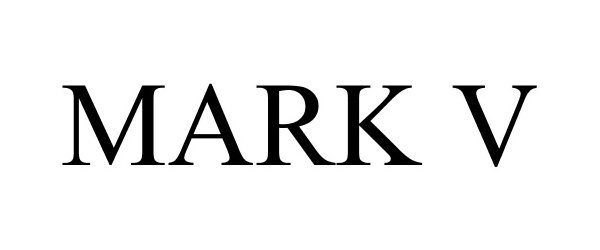 MARK V 86332055 4688459 Live/Registered |
Dee Cee Laboratories, Inc. 2014-07-09 |
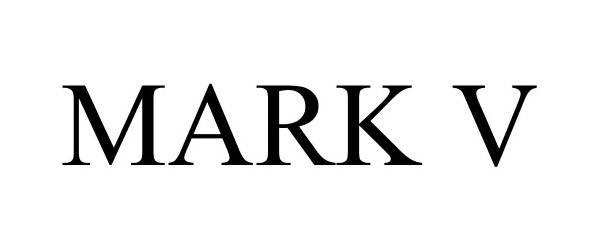 MARK V 85355681 4296187 Live/Registered |
Avery Dennison Corporation 2011-06-24 |
 MARK V 80992837 0992837 Dead/Cancelled |
Kit Manufacturing Company 0000-00-00 |
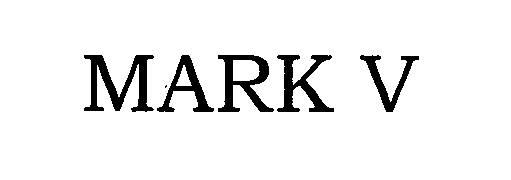 MARK V 76442661 2734473 Dead/Cancelled |
VT Halter Marine, Inc. 2002-08-22 |
 MARK V 76325520 not registered Dead/Abandoned |
Moss, Gerald 2001-10-12 |
 MARK V 75790924 2670779 Live/Registered |
MARK V PRODUCTS, INC. 1999-09-02 |
 MARK V 74685499 1971439 Dead/Cancelled |
PHILIPS ELECTRONICS NORTH AMERICA CORPORATION 1995-06-07 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.