CHLORIDE
Mercuric Thiocyanate, Colorimetry, Chloride
DILAB, INC.
The following data is part of a premarket notification filed by Dilab, Inc. with the FDA for Chloride.
Pre-market Notification Details
| Device ID | K850935 |
| 510k Number | K850935 |
| Device Name: | CHLORIDE |
| Classification | Mercuric Thiocyanate, Colorimetry, Chloride |
| Applicant | DILAB, INC. 7003 N.W. 50TH ST. Miami, FL 33166 |
| Contact | Rafael A Quevedo |
| Correspondent | Rafael A Quevedo DILAB, INC. 7003 N.W. 50TH ST. Miami, FL 33166 |
| Product Code | CHJ |
| CFR Regulation Number | 862.1170 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 1985-03-07 |
| Decision Date | 1985-06-10 |
Trademark Results [CHLORIDE]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
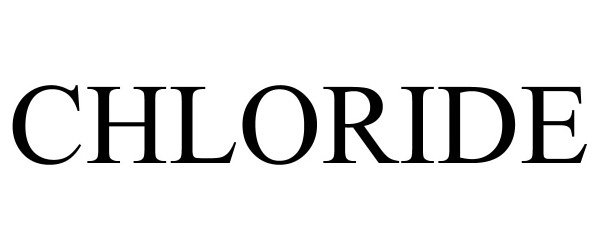 CHLORIDE 88898141 not registered Live/Pending |
Signify Holding B.V. 2020-05-02 |
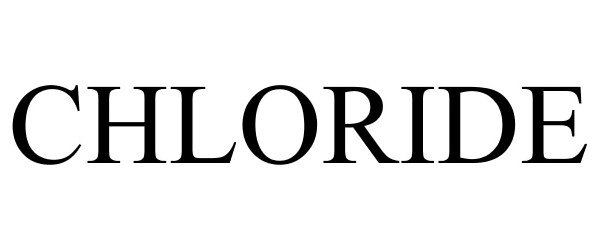 CHLORIDE 87740827 not registered Dead/Abandoned |
CHLORIDE GROUP LIMITED 2018-01-02 |
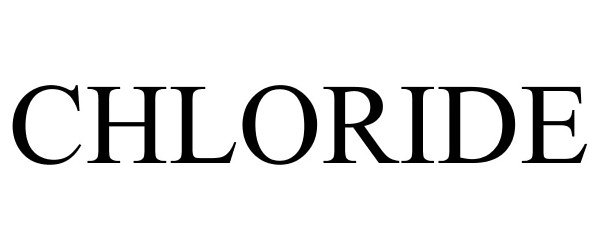 CHLORIDE 85648367 5775722 Live/Registered |
Chloride Group Limited 2012-06-11 |
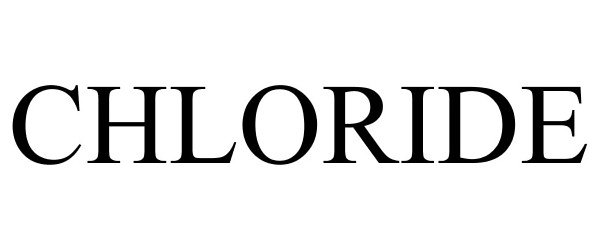 CHLORIDE 85648356 5590486 Live/Registered |
Chloride Group Limited 2012-06-11 |
 CHLORIDE 85318742 not registered Dead/Abandoned |
Exide Technologies 2011-05-11 |
 CHLORIDE 77116291 not registered Dead/Abandoned |
Exide Technologies 2007-02-26 |
 CHLORIDE 73651294 not registered Dead/Abandoned |
CHLORIDE GROUP PUBLIC LIMITED COMPANY 1987-03-25 |
 CHLORIDE 73553725 1456195 Dead/Expired |
CHLORIDE GROUP PUBLIC LIMITED COMPANY 1985-08-15 |
 CHLORIDE 73013576 1049412 Dead/Expired |
CHLORIDE GROUP LIMITED 1974-02-15 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.