NOVATHERM
System, Thermographic, Liquid Crystal, Powered (adjunctive Use)
MED TECH PRODUCTS, INC.
The following data is part of a premarket notification filed by Med Tech Products, Inc. with the FDA for Novatherm.
Pre-market Notification Details
| Device ID | K851034 |
| 510k Number | K851034 |
| Device Name: | NOVATHERM |
| Classification | System, Thermographic, Liquid Crystal, Powered (adjunctive Use) |
| Applicant | MED TECH PRODUCTS, INC. MCDERMOTT, WILL & EMERY 1850 K STREET NW Washington, DC 20006 |
| Contact | William D Appler |
| Correspondent | William D Appler MED TECH PRODUCTS, INC. MCDERMOTT, WILL & EMERY 1850 K STREET NW Washington, DC 20006 |
| Product Code | KXZ |
| CFR Regulation Number | 884.2982 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 1985-03-13 |
| Decision Date | 1985-09-27 |
Trademark Results [NOVATHERM]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
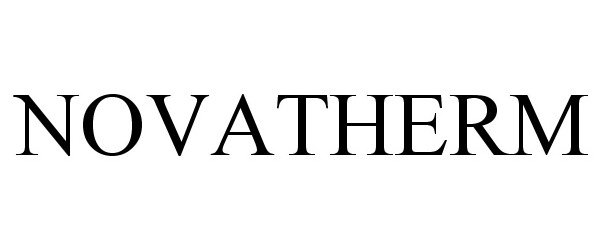 NOVATHERM 88144070 not registered Live/Pending |
Novalung GmbH 2018-10-05 |
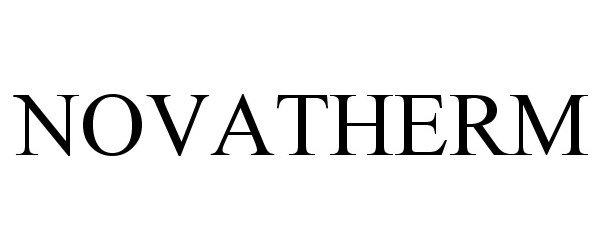 NOVATHERM 86411671 5134093 Live/Registered |
ASK CHEMICALS LLC 2014-10-01 |
 NOVATHERM 79405224 not registered Live/Pending |
Frenzelit GmbH 2024-06-24 |
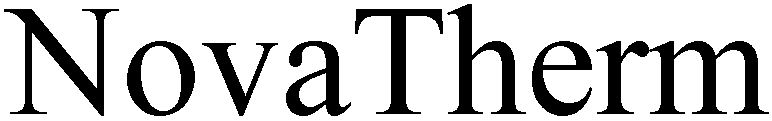 NOVATHERM 79106206 4196509 Dead/Cancelled |
novalung GmbH 2011-08-24 |
 NOVATHERM 79025344 3261294 Dead/Cancelled |
NOVAPLAST PLASTIK SANAYI VE TICARET A.S. 2006-03-28 |
 NOVATHERM 73553662 1413212 Dead/Cancelled |
MEDTECH PRODUCTS, INC. 1985-08-15 |
 NOVATHERM 73286177 not registered Dead/Abandoned |
Gebruder Ditzel GmbH 1980-11-17 |
 NOVATHERM 72423364 0973213 Dead/Expired |
NOVAGARD CORPORATION 1972-05-04 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.