INSTA-GLAZE
Agent, Polishing, Abrasive, Oral Cavity
GEORGE TAUB PRODUCTS & FUSION CO., INC.
The following data is part of a premarket notification filed by George Taub Products & Fusion Co., Inc. with the FDA for Insta-glaze.
Pre-market Notification Details
| Device ID | K854588 |
| 510k Number | K854588 |
| Device Name: | INSTA-GLAZE |
| Classification | Agent, Polishing, Abrasive, Oral Cavity |
| Applicant | GEORGE TAUB PRODUCTS & FUSION CO., INC. 277 NEW YORK AVE. Jersey City, NJ 07307 |
| Contact | Lawrence D Taub |
| Correspondent | Lawrence D Taub GEORGE TAUB PRODUCTS & FUSION CO., INC. 277 NEW YORK AVE. Jersey City, NJ 07307 |
| Product Code | EJR |
| CFR Regulation Number | 872.6030 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 1985-11-18 |
| Decision Date | 1986-02-04 |
NIH GUDID Devices
| Device Identifier | submissionNumber | Supplement |
|---|---|---|
| D7799061050 | K854588 | 000 |
| D7799061040 | K854588 | 000 |
Trademark Results [INSTA-GLAZE]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
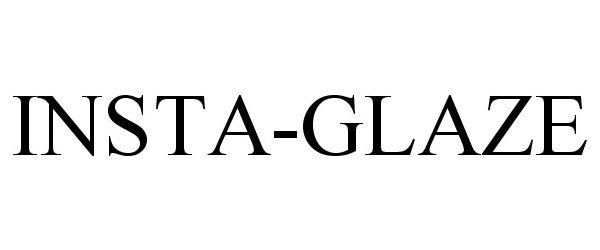 INSTA-GLAZE 78910508 3233201 Dead/Cancelled |
George Taub Products and Fusion Co., Inc. 2006-06-16 |
 INSTA-GLAZE 73420716 1289708 Dead/Cancelled |
George Taub Products and Fusion Company, Inc. 1983-04-08 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.