LM 1000
Unit, Cryosurgical, Accessories
LEISEGANG MEDICAL, INC.
The following data is part of a premarket notification filed by Leisegang Medical, Inc. with the FDA for Lm 1000.
Pre-market Notification Details
| Device ID | K854767 |
| 510k Number | K854767 |
| Device Name: | LM 1000 |
| Classification | Unit, Cryosurgical, Accessories |
| Applicant | LEISEGANG MEDICAL, INC. 8801 S.W. 129 STREET Miami, FL 33176 |
| Contact | Douglas Kwart |
| Correspondent | Douglas Kwart LEISEGANG MEDICAL, INC. 8801 S.W. 129 STREET Miami, FL 33176 |
| Product Code | GEH |
| CFR Regulation Number | 878.4350 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 1985-11-27 |
| Decision Date | 1986-01-24 |
NIH GUDID Devices
| Device Identifier | submissionNumber | Supplement |
|---|---|---|
| 60888937009974 | K854767 | 000 |
| 00888937000122 | K854767 | 000 |
| 00888937000139 | K854767 | 000 |
| 00888937000146 | K854767 | 000 |
| 00888937000153 | K854767 | 000 |
| 00888937000160 | K854767 | 000 |
| 00888937000177 | K854767 | 000 |
| 00888937000184 | K854767 | 000 |
| 00888937000191 | K854767 | 000 |
| 00888937000207 | K854767 | 000 |
| 00888937000214 | K854767 | 000 |
| 00888937000221 | K854767 | 000 |
| 00888937000238 | K854767 | 000 |
| 00888937000245 | K854767 | 000 |
| 00888937000252 | K854767 | 000 |
| 60888937009967 | K854767 | 000 |
| 00888937000108 | K854767 | 000 |
Trademark Results [LM 1000]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
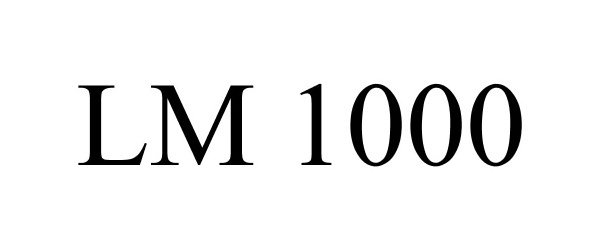 LM 1000 87612450 not registered Live/Pending |
Lockheed Martin Corporation 2017-09-18 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.