MIRALITE
Laser, Ophthalmic
MIRA, INC.
The following data is part of a premarket notification filed by Mira, Inc. with the FDA for Miralite.
Pre-market Notification Details
| Device ID | K863246 |
| 510k Number | K863246 |
| Device Name: | MIRALITE |
| Classification | Laser, Ophthalmic |
| Applicant | MIRA, INC. 87 RUMFORD AVE. Waltham, MA 02453 -3846 |
| Contact | Mark W Furlong |
| Correspondent | Mark W Furlong MIRA, INC. 87 RUMFORD AVE. Waltham, MA 02453 -3846 |
| Product Code | HQF |
| CFR Regulation Number | 886.4390 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 1986-08-22 |
| Decision Date | 1986-10-31 |
Trademark Results [MIRALITE]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 MIRALITE 86019975 not registered Dead/Abandoned |
Foamtec International Co., Ltd. 2013-07-25 |
 MIRALITE 76691625 not registered Dead/Abandoned |
Remicalm LLC 2008-07-28 |
 MIRALITE 74159333 1676646 Dead/Cancelled |
Miralite Communications, Inc. 1991-04-22 |
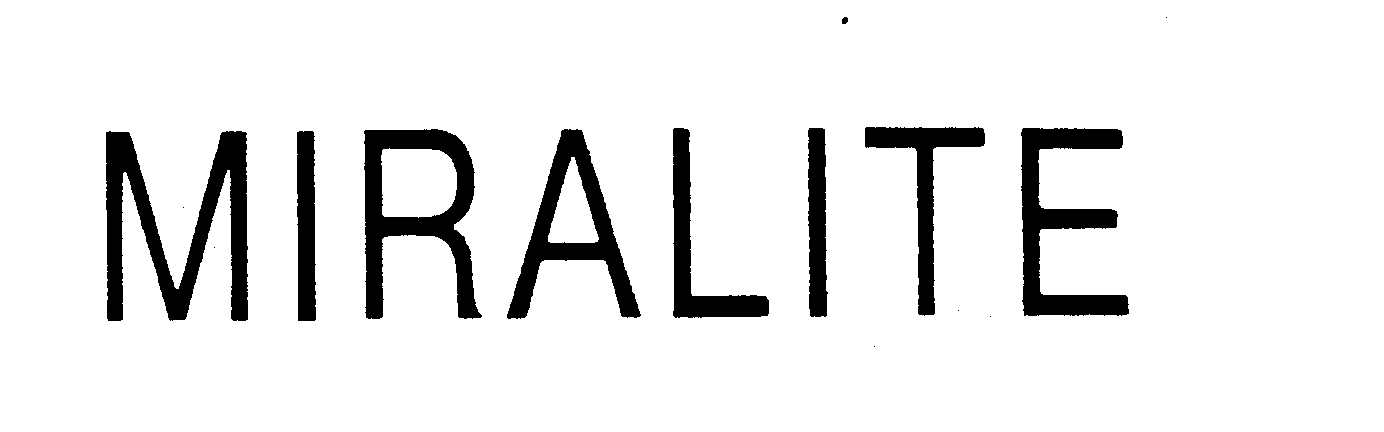 MIRALITE 73838158 1609947 Dead/Cancelled |
DOW CORNING CORPORATION 1989-11-13 |
 MIRALITE 73488177 1327806 Dead/Cancelled |
Pierce & Stevens Chemical Corp. 1984-07-02 |
 MIRALITE 73396595 1295549 Dead/Cancelled |
Edwards; James G. 1982-09-30 |
 MIRALITE 73305398 1195606 Dead/Cancelled |
Miralite 1981-04-13 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.