LIGHT GREEN
Stains, Microbiologic
REMEL CO.
The following data is part of a premarket notification filed by Remel Co. with the FDA for Light Green.
Pre-market Notification Details
| Device ID | K872185 |
| 510k Number | K872185 |
| Device Name: | LIGHT GREEN |
| Classification | Stains, Microbiologic |
| Applicant | REMEL CO. 12076 SANTA FE DR. Lenexa, KS 66215 |
| Contact | James G Baxendale |
| Correspondent | James G Baxendale REMEL CO. 12076 SANTA FE DR. Lenexa, KS 66215 |
| Product Code | JTS |
| CFR Regulation Number | 864.1850 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 1987-06-08 |
| Decision Date | 1987-07-29 |
Trademark Results [LIGHT GREEN]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
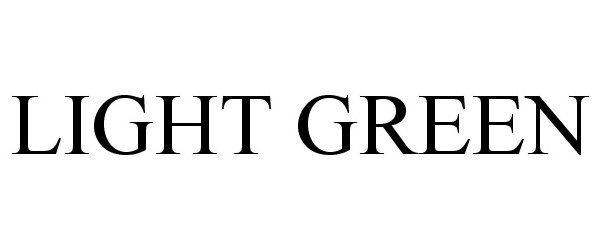 LIGHT GREEN 86194535 4710614 Live/Registered |
Marin Clean Energy 2014-02-14 |
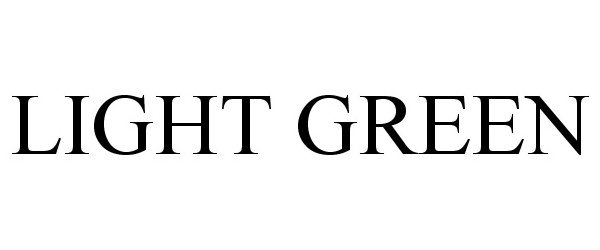 LIGHT GREEN 77410315 not registered Dead/Abandoned |
Thor Tech, Inc. 2008-02-29 |
 LIGHT GREEN 77168190 not registered Dead/Abandoned |
Denton, Benton & Steele, LLC 2007-04-27 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.