HOT WHEELS
Wheelchair, Powered
EVEREST & JENNINGS, INC.
The following data is part of a premarket notification filed by Everest & Jennings, Inc. with the FDA for Hot Wheels.
Pre-market Notification Details
| Device ID | K873895 |
| 510k Number | K873895 |
| Device Name: | HOT WHEELS |
| Classification | Wheelchair, Powered |
| Applicant | EVEREST & JENNINGS, INC. 3233 E MISSION OAKS BLVD. Camarillo, CA 93010 |
| Contact | Murray E Smith |
| Correspondent | Murray E Smith EVEREST & JENNINGS, INC. 3233 E MISSION OAKS BLVD. Camarillo, CA 93010 |
| Product Code | ITI |
| CFR Regulation Number | 890.3860 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 1987-09-24 |
| Decision Date | 1987-11-24 |
Trademark Results [HOT WHEELS]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 HOT WHEELS 98472208 not registered Live/Pending |
MATTEL, INC. 2024-03-28 |
 HOT WHEELS 98310189 not registered Live/Pending |
MATTEL, INC. 2023-12-12 |
 HOT WHEELS 97253187 not registered Live/Pending |
MATTEL, INC. 2022-02-04 |
 HOT WHEELS 90980214 not registered Live/Pending |
MATTEL, INC. 2021-02-11 |
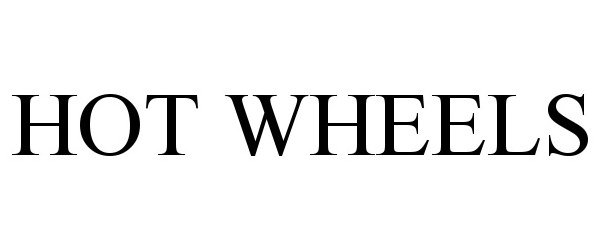 HOT WHEELS 90977670 not registered Live/Pending |
MATTEL, INC. 2021-01-20 |
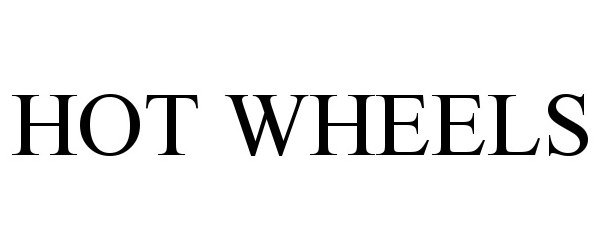 HOT WHEELS 90524383 not registered Live/Pending |
MATTEL, INC. 2021-02-11 |
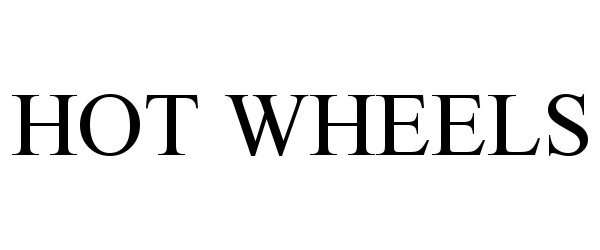 HOT WHEELS 90476497 not registered Live/Pending |
MATTEL, INC. 2021-01-20 |
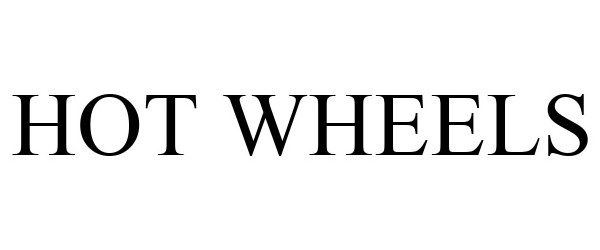 HOT WHEELS 88934643 not registered Live/Pending |
MATTEL, INC. 2020-05-27 |
 HOT WHEELS 88934638 not registered Live/Pending |
MATTEL, INC. 2020-05-27 |
 HOT WHEELS 88044345 not registered Live/Pending |
MATTEL, INC. 2018-07-19 |
 HOT WHEELS 87600899 5571920 Live/Registered |
MATTEL, INC. 2017-09-08 |
 HOT WHEELS 87338928 not registered Live/Pending |
MATTEL, INC. 2017-02-16 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.