AQUADERM
Tape And Bandage, Adhesive
LAIVAN CORP.
The following data is part of a premarket notification filed by Laivan Corp. with the FDA for Aquaderm.
Pre-market Notification Details
| Device ID | K885205 |
| 510k Number | K885205 |
| Device Name: | AQUADERM |
| Classification | Tape And Bandage, Adhesive |
| Applicant | LAIVAN CORP. 49 FRONT ST. P.O. BOX 175 East Rockaway, NY 11518 |
| Contact | Joel Martz |
| Correspondent | Joel Martz LAIVAN CORP. 49 FRONT ST. P.O. BOX 175 East Rockaway, NY 11518 |
| Product Code | KGX |
| CFR Regulation Number | 880.5240 [🔎] |
| Decision | Substantially Equivalent For Some Indications (SN) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 1988-12-19 |
| Decision Date | 1989-03-29 |
Trademark Results [AQUADERM]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 AQUADERM 88320615 not registered Live/Pending |
LAPKO Inc. 2019-02-28 |
 AQUADERM 78777758 3180503 Dead/Cancelled |
Lanxess Deutschland GmbH 2005-12-21 |
 AQUADERM 74663213 1986866 Dead/Cancelled |
Bayer Aktiengesellschaft 1995-04-19 |
 AQUADERM 73757881 1578499 Dead/Cancelled |
BAKER CUMMINS LABORATORIES, INC. 1988-10-17 |
 AQUADERM 73595621 not registered Dead/Abandoned |
LABORATOIRES ED. FROMONT 1986-04-28 |
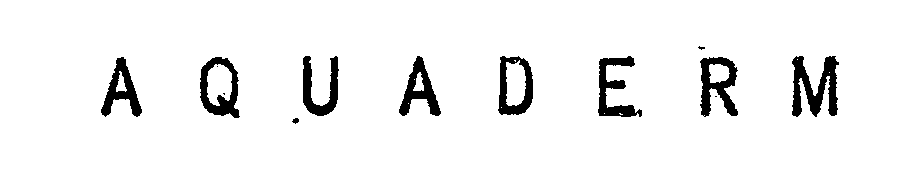 AQUADERM 72183084 0779351 Dead/Expired |
MENLEY & JAMES LABORATORIES, LTD. 1963-12-16 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.