POLARON
Aid, Vision, Electronic, Battery-powered
NURION INDUSTRIES
The following data is part of a premarket notification filed by Nurion Industries with the FDA for Polaron.
Pre-market Notification Details
| Device ID | K902187 |
| 510k Number | K902187 |
| Device Name: | POLARON |
| Classification | Aid, Vision, Electronic, Battery-powered |
| Applicant | NURION INDUSTRIES STATION SQUARE THREE Paoli, PA 19301 |
| Contact | William P Rosen |
| Correspondent | William P Rosen NURION INDUSTRIES STATION SQUARE THREE Paoli, PA 19301 |
| Product Code | HPG |
| CFR Regulation Number | 886.5900 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 1990-05-14 |
| Decision Date | 1990-08-08 |
Trademark Results [POLARON]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
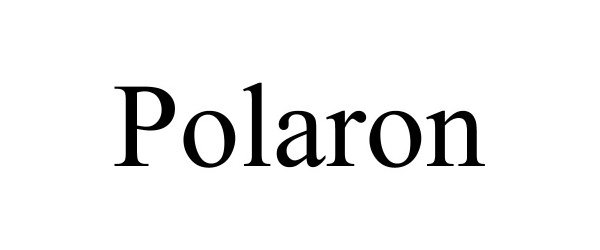 POLARON 88710835 not registered Live/Pending |
Blue Heron Scientific, LLC 2019-11-30 |
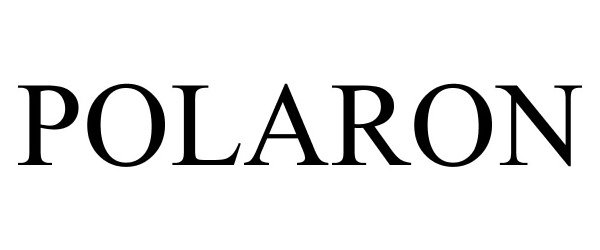 POLARON 88157570 not registered Live/Pending |
POLARON CAPITAL LLC 2018-10-16 |
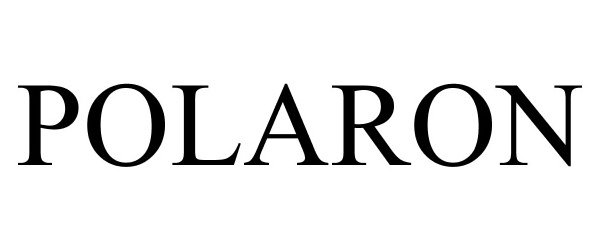 POLARON 87269110 5303427 Live/Registered |
Hamilton Associates, Inc. 2016-12-14 |
 POLARON 78062644 not registered Dead/Abandoned |
VG Systems Limited 2001-05-09 |
 POLARON 74619344 not registered Dead/Abandoned |
Polaron International Inc. 1995-01-09 |
 POLARON 72263719 0843580 Dead/Expired |
WARREN-TEED PHARMACEUTICALS INC. 1967-01-31 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.