STERI-SHIELD
Hood, Surgical
BIOMEDICAL DEVICES, INC.
The following data is part of a premarket notification filed by Biomedical Devices, Inc. with the FDA for Steri-shield.
Pre-market Notification Details
| Device ID | K903533 |
| 510k Number | K903533 |
| Device Name: | STERI-SHIELD |
| Classification | Hood, Surgical |
| Applicant | BIOMEDICAL DEVICES, INC. 1501 E. CHAPMAN, SUITE 356 Fullerton, CA 92631 |
| Contact | Nick Herbert |
| Correspondent | Nick Herbert BIOMEDICAL DEVICES, INC. 1501 E. CHAPMAN, SUITE 356 Fullerton, CA 92631 |
| Product Code | FXY |
| CFR Regulation Number | 878.4040 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 1990-08-06 |
| Decision Date | 1990-10-19 |
Trademark Results [STERI-SHIELD]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
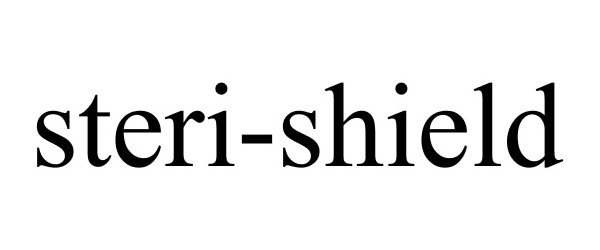 STERI-SHIELD 88090848 5715091 Live/Registered |
Steri-Shield Inc. 2018-08-23 |
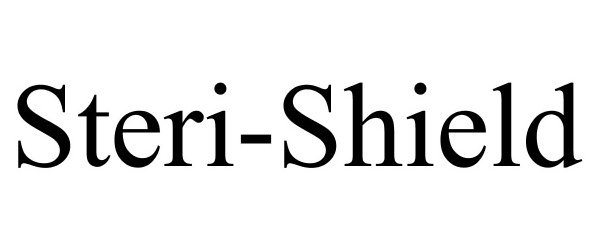 STERI-SHIELD 77027919 3356891 Dead/Cancelled |
Portola Packaging, Inc. 2006-10-24 |
 STERI-SHIELD 74568273 1950561 Live/Registered |
Dentex Ltd. Partnership 1994-08-31 |
 STERI-SHIELD 74057722 1777108 Live/Registered |
BIO-MEDICAL DEVICES, INC. 1990-05-10 |
 STERI-SHIELD 73799288 1638981 Dead/Cancelled |
COOK (CANADA) INC. 1989-05-11 |
 STERI-SHIELD 73572963 1402121 Dead/Cancelled |
HENSLEY AND SWANSON PARTNERS 1985-12-10 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.