HIGH RISK
Latex Patient Examination Glove
MEDICAL RESOURCES, LTD.
The following data is part of a premarket notification filed by Medical Resources, Ltd. with the FDA for High Risk.
Pre-market Notification Details
| Device ID | K903559 |
| 510k Number | K903559 |
| Device Name: | HIGH RISK |
| Classification | Latex Patient Examination Glove |
| Applicant | MEDICAL RESOURCES, LTD. 520 WEST BRANNEN RD. Lakeland, FL 33813 |
| Contact | Richard H Ghenn |
| Correspondent | Richard H Ghenn MEDICAL RESOURCES, LTD. 520 WEST BRANNEN RD. Lakeland, FL 33813 |
| Product Code | LYY |
| CFR Regulation Number | 880.6250 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 1990-08-08 |
| Decision Date | 1990-12-21 |
Trademark Results [HIGH RISK]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 HIGH RISK 98809826 not registered Live/Pending |
Duncan, Thomas 2024-10-18 |
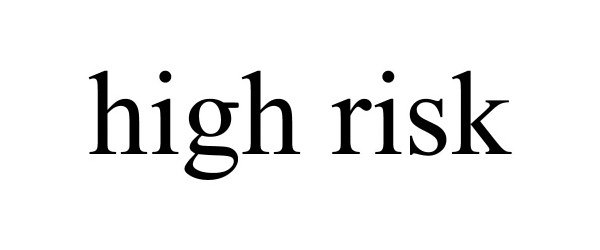 HIGH RISK 90087067 not registered Live/Pending |
Feingold, Michael 2020-07-31 |
 HIGH RISK 75595350 2320959 Dead/Cancelled |
Bolen, Frederick L. 1998-11-24 |
 HIGH RISK 75226492 2116375 Dead/Cancelled |
Serpent's Tail Ltd. 1997-01-16 |
 HIGH RISK 74132496 1709965 Dead/Cancelled |
Climax Paper Converters, Inc. 1991-01-22 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.