FLEXISYS
Powered Laser Surgical Instrument
DORNIER MEDTECH AMERICA, INC.
The following data is part of a premarket notification filed by Dornier Medtech America, Inc. with the FDA for Flexisys.
Pre-market Notification Details
| Device ID | K904149 |
| 510k Number | K904149 |
| Device Name: | FLEXISYS |
| Classification | Powered Laser Surgical Instrument |
| Applicant | DORNIER MEDTECH AMERICA, INC. 824 LIVINGSTON COURT Marietta, GA 30067 |
| Contact | Robert E Yocher |
| Correspondent | Robert E Yocher DORNIER MEDTECH AMERICA, INC. 824 LIVINGSTON COURT Marietta, GA 30067 |
| Product Code | GEX |
| CFR Regulation Number | 878.4810 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 1990-09-07 |
| Decision Date | 1990-12-04 |
Trademark Results [FLEXISYS]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
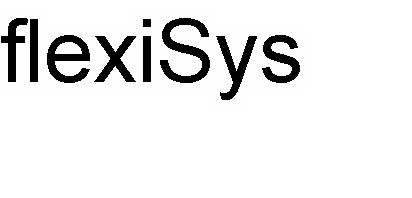 FLEXISYS 78496411 3203415 Dead/Cancelled |
Dornier MedTech GmbH 2004-10-07 |
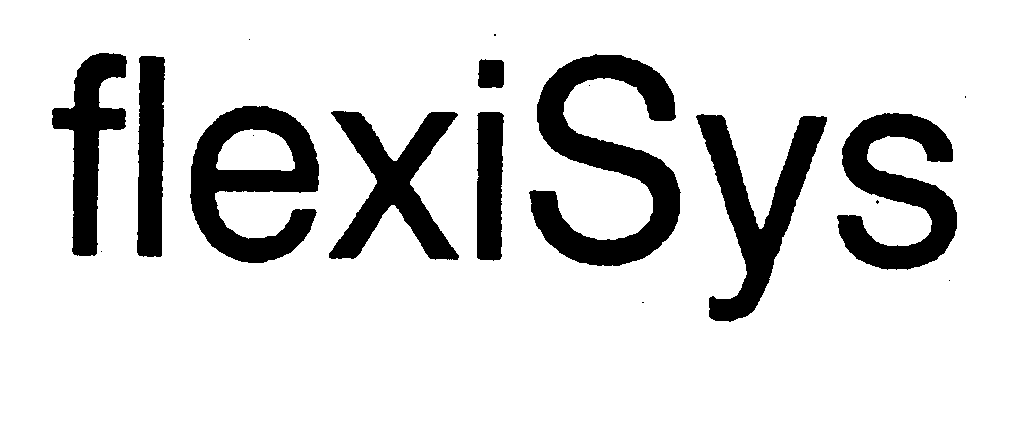 FLEXISYS 74065780 1888346 Dead/Cancelled |
Messerschmitt-Bolkow-Blohm GmbH 1990-06-04 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.