KWIKMIX
Set, I.v. Fluid Transfer
BAXA CORP., SUB. OF COOK GROUP, INC.
The following data is part of a premarket notification filed by Baxa Corp., Sub. Of Cook Group, Inc. with the FDA for Kwikmix.
Pre-market Notification Details
| Device ID | K904225 |
| 510k Number | K904225 |
| Device Name: | KWIKMIX |
| Classification | Set, I.v. Fluid Transfer |
| Applicant | BAXA CORP., SUB. OF COOK GROUP, INC. 13760 E. ARAPAHOE RD. Englewood, CO 80112 |
| Contact | Mary Steggall |
| Correspondent | Mary Steggall BAXA CORP., SUB. OF COOK GROUP, INC. 13760 E. ARAPAHOE RD. Englewood, CO 80112 |
| Product Code | LHI |
| CFR Regulation Number | 880.5440 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 1990-09-13 |
| Decision Date | 1990-11-05 |
Trademark Results [KWIKMIX]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
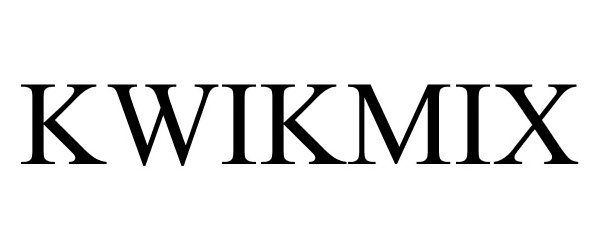 KWIKMIX 87465759 not registered Live/Pending |
Milk Specialties Company 2017-05-26 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.