STETHOSCOPE
Stethoscope, Manual
KIMBALL INDUSTRIES CO., LTD.
The following data is part of a premarket notification filed by Kimball Industries Co., Ltd. with the FDA for Stethoscope.
Pre-market Notification Details
| Device ID | K904547 |
| 510k Number | K904547 |
| Device Name: | STETHOSCOPE |
| Classification | Stethoscope, Manual |
| Applicant | KIMBALL INDUSTRIES CO., LTD. C/O HEALTH PRO TECH, INC. 113 ROBINWOOD DRIVE Shirley, NY 11967 |
| Contact | Rose Daleo |
| Correspondent | Rose Daleo KIMBALL INDUSTRIES CO., LTD. C/O HEALTH PRO TECH, INC. 113 ROBINWOOD DRIVE Shirley, NY 11967 |
| Product Code | LDE |
| CFR Regulation Number | 870.1875 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 1990-10-03 |
| Decision Date | 1991-03-21 |
Trademark Results [STETHOSCOPE]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 STETHOSCOPE 78066577 not registered Dead/Abandoned |
Stethoscope Manufacturers Association 2001-05-31 |
 STETHOSCOPE 78066561 not registered Dead/Abandoned |
Stethoscope Manufacturers Association 2001-05-31 |
 STETHOSCOPE 78066547 not registered Dead/Abandoned |
Emergency Medical Technician Student Association 2001-05-31 |
 STETHOSCOPE 77286626 4053311 Dead/Cancelled |
O'Gorman, Dr. Ronald 2007-09-23 |
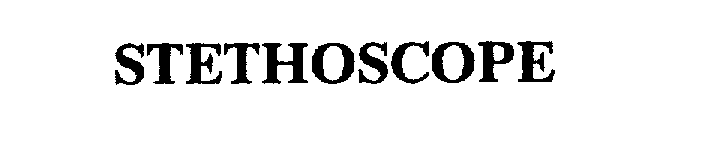 STETHOSCOPE 75642019 2332002 Dead/Cancelled |
Real-Time Innovations, Inc. 1999-02-17 |
 STETHOSCOPE 74225959 1709861 Dead/Cancelled |
Real-Time Innovations, Inc. 1991-11-26 |
 STETHOSCOPE 74122708 1708072 Dead/Cancelled |
National Association of Residents and Interns, Inc. 1990-12-12 |
 STETHOSCOPE 74122666 1717980 Dead/Cancelled |
National Association of Residents and Interns, Inc. 1990-12-12 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.