OPTYX
Shunt, Central Nervous System And Components
BAXTER HEALTHCARE CORP.
The following data is part of a premarket notification filed by Baxter Healthcare Corp. with the FDA for Optyx.
Pre-market Notification Details
| Device ID | K904883 |
| 510k Number | K904883 |
| Device Name: | OPTYX |
| Classification | Shunt, Central Nervous System And Components |
| Applicant | BAXTER HEALTHCARE CORP. 17221 RED HILL AVE. PO BOX 11150 Irvine, CA 92614 -5627 |
| Contact | Jack P Douglas |
| Correspondent | Jack P Douglas BAXTER HEALTHCARE CORP. 17221 RED HILL AVE. PO BOX 11150 Irvine, CA 92614 -5627 |
| Product Code | JXG |
| CFR Regulation Number | 882.5550 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 1990-10-26 |
| Decision Date | 1991-07-24 |
Trademark Results [OPTYX]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 OPTYX 87597518 not registered Live/Pending |
Kantro, Scott 2017-09-06 |
 OPTYX 78721816 3186261 Dead/Cancelled |
McKesson Corporation 2005-09-27 |
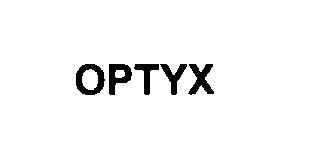 OPTYX 76262858 2652624 Live/Registered |
Key Technology, Inc. 2001-05-25 |
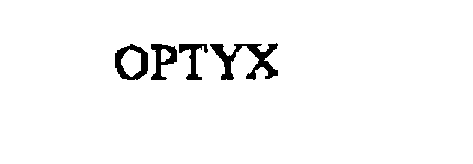 OPTYX 76185160 2562543 Dead/Cancelled |
EBONITE HOLDING, INC. 2000-12-21 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.