HAZEL
Bed, Flotation Therapy, Powered
KINETIC CONCEPTS, INC.
The following data is part of a premarket notification filed by Kinetic Concepts, Inc. with the FDA for Hazel.
Pre-market Notification Details
| Device ID | K912855 |
| 510k Number | K912855 |
| Device Name: | HAZEL |
| Classification | Bed, Flotation Therapy, Powered |
| Applicant | KINETIC CONCEPTS, INC. P.O. BOX 8588 San Antonio, TX 78208 |
| Contact | William H Quirk |
| Correspondent | William H Quirk KINETIC CONCEPTS, INC. P.O. BOX 8588 San Antonio, TX 78208 |
| Product Code | IOQ |
| CFR Regulation Number | 890.5170 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 1991-06-28 |
| Decision Date | 1991-09-06 |
Trademark Results [HAZEL]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 HAZEL 98554264 not registered Live/Pending |
Hazel AI Technologies, Inc. 2024-05-16 |
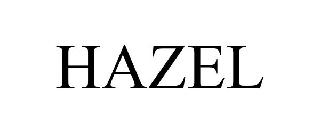 HAZEL 98541433 not registered Live/Pending |
Parlytix, Inc. 2024-05-09 |
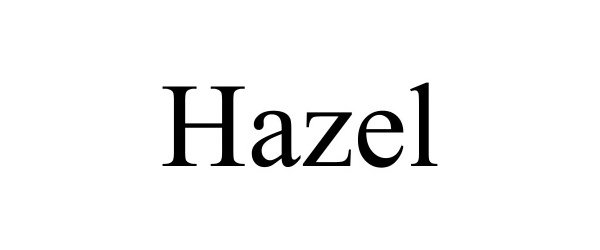 HAZEL 98449202 not registered Live/Pending |
Joie International Co., Limited 2024-03-14 |
 HAZEL 97929205 not registered Live/Pending |
Bad Dragon Enterprises, Inc. 2023-05-10 |
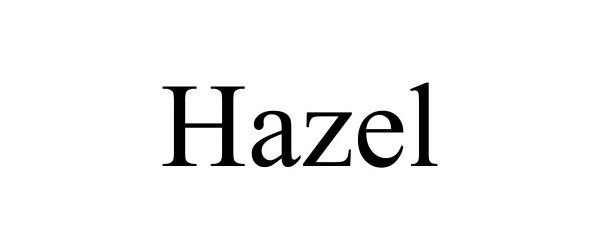 HAZEL 97879820 not registered Live/Pending |
JT III LLC 2023-04-09 |
 HAZEL 97762064 not registered Live/Pending |
Value Tree Retail LLP 2023-01-20 |
 HAZEL 97466409 not registered Live/Pending |
Juno Diagnostics, Inc. 2022-06-20 |
 HAZEL 90669440 not registered Live/Pending |
Qu Qi 2021-04-25 |
 HAZEL 90634196 not registered Live/Pending |
Walmart Apollo, LLC 2021-04-09 |
 HAZEL 90547039 not registered Live/Pending |
Walmart Apollo, LLC 2021-02-25 |
 HAZEL 90351941 not registered Live/Pending |
Hound Labs, Inc. 2020-12-01 |
 HAZEL 90188866 not registered Live/Pending |
Hazel Technologies, Inc. 2020-09-17 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.