RHYTHM
Analyzer, Spectrum, Electroencephalogram Signal
STELLATE SYSTEMS
The following data is part of a premarket notification filed by Stellate Systems with the FDA for Rhythm.
Pre-market Notification Details
| Device ID | K912938 |
| 510k Number | K912938 |
| Device Name: | RHYTHM |
| Classification | Analyzer, Spectrum, Electroencephalogram Signal |
| Applicant | STELLATE SYSTEMS 345 VICTORIA AVE., SUITE 300 Westmount, Quebec, CA H3z 2n2 |
| Contact | Gotman |
| Correspondent | Gotman STELLATE SYSTEMS 345 VICTORIA AVE., SUITE 300 Westmount, Quebec, CA H3z 2n2 |
| Product Code | GWS |
| CFR Regulation Number | 882.1420 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 1991-07-05 |
| Decision Date | 1991-07-30 |
Trademark Results [RHYTHM]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 RHYTHM 98892245 not registered Live/Pending |
Ojai Retinal Technology, LLC 2024-12-09 |
 RHYTHM 98729407 not registered Live/Pending |
Chen, Youzhi 2024-09-02 |
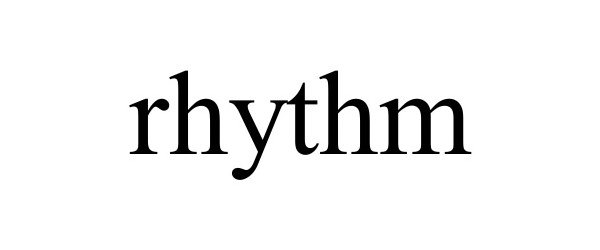 RHYTHM 98679029 not registered Live/Pending |
Rhythm Hydration Co. 2024-08-01 |
 RHYTHM 98660178 not registered Live/Pending |
Rhythm Healthcare, LLC 2024-07-22 |
 RHYTHM 98660150 not registered Live/Pending |
Rhythm Healthcare, LLC 2024-07-22 |
 RHYTHM 98381609 not registered Live/Pending |
Apollo Neuroscience, Inc. 2024-01-30 |
 RHYTHM 98324135 not registered Live/Pending |
ENOVATE MEDICAL, LLC 2023-12-20 |
 RHYTHM 98264462 not registered Live/Pending |
CardioOne Inc. 2023-11-10 |
 RHYTHM 97908030 not registered Live/Pending |
Skin, Mind, Body Essentials LLC 2023-04-26 |
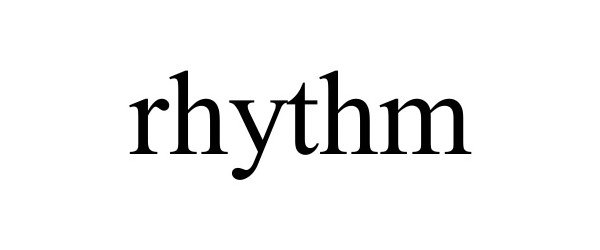 RHYTHM 97535227 not registered Live/Pending |
rhythm care, llc 2022-08-04 |
 RHYTHM 97461527 not registered Live/Pending |
Twin Peaks Winery, Inc. 2022-06-16 |
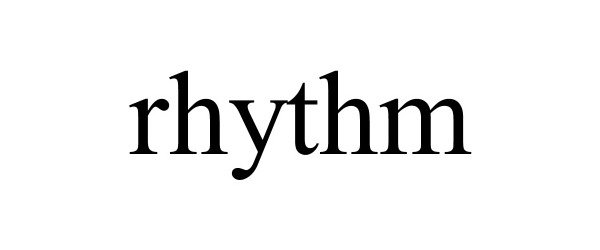 RHYTHM 97256859 not registered Live/Pending |
Gui Zuxing 2022-02-07 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.