MINI-MILL
File, Bone, Surgical
BIOMEDICAL COMPOSITES, LTD.
The following data is part of a premarket notification filed by Biomedical Composites, Ltd. with the FDA for Mini-mill.
Pre-market Notification Details
| Device ID | K914035 |
| 510k Number | K914035 |
| Device Name: | MINI-MILL |
| Classification | File, Bone, Surgical |
| Applicant | BIOMEDICAL COMPOSITES, LTD. 5464 CARPINTERIA AVE. #219 Carpinteria, CA 93013 |
| Contact | Richard Mullarky |
| Correspondent | Richard Mullarky BIOMEDICAL COMPOSITES, LTD. 5464 CARPINTERIA AVE. #219 Carpinteria, CA 93013 |
| Product Code | EMI |
| CFR Regulation Number | 872.4565 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 1991-09-10 |
| Decision Date | 1992-03-02 |
Trademark Results [MINI-MILL]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
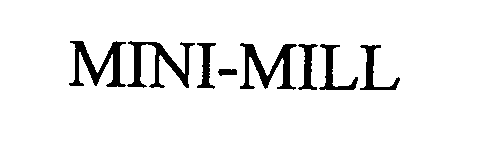 MINI-MILL 76474739 not registered Dead/Abandoned |
Jeffrey Specialty Equipment Corporation 2002-12-13 |
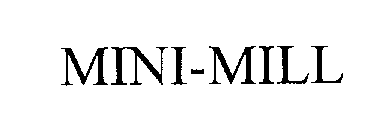 MINI-MILL 76205275 not registered Dead/Abandoned |
WOODSTOCK INTERNATIONAL, INC. 2001-02-06 |
 MINI-MILL 72383080 0964914 Dead/Expired |
PENNSYLVANIA ENGINEERING CORPORATION 1971-02-05 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.