SIL-K
Elastomer, Silicone, For Scar Management
DEGANIA SILICONE, LTD.
The following data is part of a premarket notification filed by Degania Silicone, Ltd. with the FDA for Sil-k.
Pre-market Notification Details
| Device ID | K914701 |
| 510k Number | K914701 |
| Device Name: | SIL-K |
| Classification | Elastomer, Silicone, For Scar Management |
| Applicant | DEGANIA SILICONE, LTD. DEGANIA BET Emek Hayarden, IL 15130 |
| Contact | Bette Lubin |
| Correspondent | Bette Lubin DEGANIA SILICONE, LTD. DEGANIA BET Emek Hayarden, IL 15130 |
| Product Code | MDA |
| CFR Regulation Number | 878.4025 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 1991-10-18 |
| Decision Date | 1992-01-31 |
Trademark Results [SIL-K]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
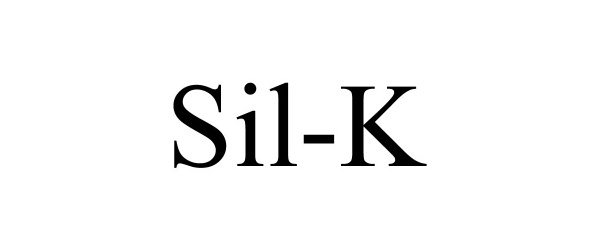 SIL-K 88770969 not registered Live/Pending |
Solutech Pharmaceuticals, LLC 2020-01-23 |
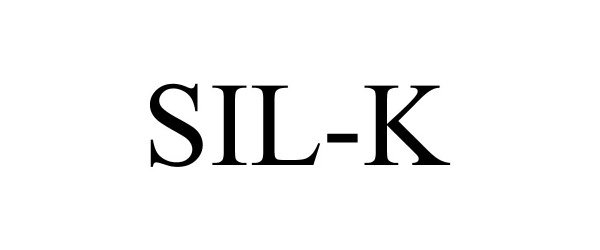 SIL-K 88671404 not registered Live/Pending |
Vivera Pharmaceuticals, Inc. 2019-10-28 |
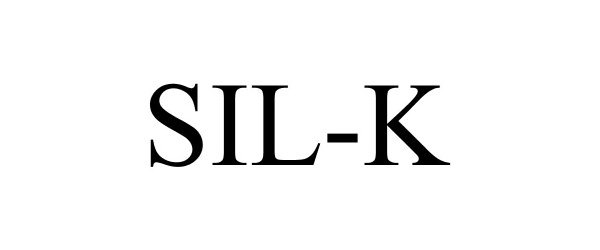 SIL-K 87041965 5179650 Live/Registered |
Bio Huma Netics, Inc. 2016-05-18 |
 SIL-K 74250562 1911019 Dead/Cancelled |
Degania Silicone Ltd. 1992-02-28 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.