AUTOLOADER
Station, Pipetting And Diluting, For Clinical Use
INNOVATIVE MEDICAL SYSTEMS, INC.
The following data is part of a premarket notification filed by Innovative Medical Systems, Inc. with the FDA for Autoloader.
Pre-market Notification Details
| Device ID | K921005 |
| 510k Number | K921005 |
| Device Name: | AUTOLOADER |
| Classification | Station, Pipetting And Diluting, For Clinical Use |
| Applicant | INNOVATIVE MEDICAL SYSTEMS, INC. 55 STEAM WHISTLE DR. Ivyland, PA 18974 |
| Contact | Richard Crymes |
| Correspondent | Richard Crymes INNOVATIVE MEDICAL SYSTEMS, INC. 55 STEAM WHISTLE DR. Ivyland, PA 18974 |
| Product Code | JQW |
| CFR Regulation Number | 862.2750 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 1992-03-03 |
| Decision Date | 1992-04-29 |
Trademark Results [AUTOLOADER]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 AUTOLOADER 97824650 not registered Live/Pending |
Wing Aviation LLC 2023-03-06 |
 AUTOLOADER 97824648 not registered Live/Pending |
Wing Aviation LLC 2023-03-06 |
 AUTOLOADER 85146478 3988878 Live/Registered |
EAGLEMARK INDUSTRIES INC. 2010-10-06 |
 AUTOLOADER 78191216 2802386 Dead/Cancelled |
SICOM INDUSTRIES LTD. 2002-12-04 |
 AUTOLOADER 77106470 3293463 Dead/Cancelled |
Malone Paddle Gear, LLC 2007-02-13 |
 AUTOLOADER 74481383 not registered Dead/Abandoned |
Media Vision Technology, Inc. 1994-01-24 |
 AUTOLOADER 74375585 1822352 Dead/Cancelled |
AEC, Inc. 1993-04-05 |
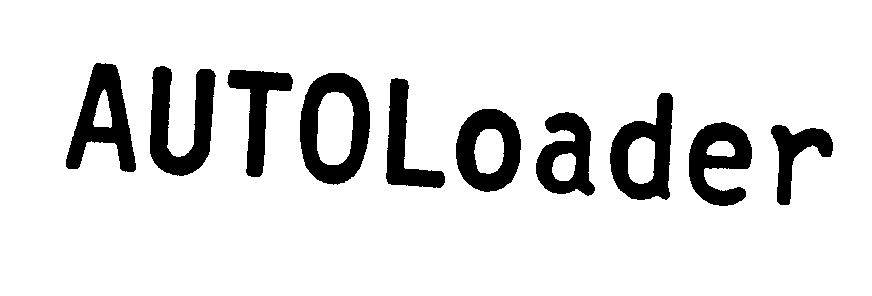 AUTOLOADER 74231450 not registered Dead/Abandoned |
WangDAT, Inc. 1991-12-17 |
 AUTOLOADER 73681288 1553355 Dead/Cancelled |
BRISTOL ASSOCIATES, INC. 1987-08-14 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.