ULTRABLADE
Scalpel, One-piece
MICRON TECHNOLOGY, INC.
The following data is part of a premarket notification filed by Micron Technology, Inc. with the FDA for Ultrablade.
Pre-market Notification Details
| Device ID | K921167 |
| 510k Number | K921167 |
| Device Name: | ULTRABLADE |
| Classification | Scalpel, One-piece |
| Applicant | MICRON TECHNOLOGY, INC. CENTENNIAL CREEK OFFICE PARK 2975 VALMONT AVE., SUITE 310 Boulder, CO 80301 |
| Contact | Richard Rosenthal |
| Correspondent | Richard Rosenthal MICRON TECHNOLOGY, INC. CENTENNIAL CREEK OFFICE PARK 2975 VALMONT AVE., SUITE 310 Boulder, CO 80301 |
| Product Code | GDX |
| CFR Regulation Number | 878.4800 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 1992-03-11 |
| Decision Date | 1992-07-30 |
Trademark Results [ULTRABLADE]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ULTRABLADE 98622160 not registered Live/Pending |
Fiskars Finland Oy Ab 2024-06-27 |
 ULTRABLADE 88228391 not registered Live/Pending |
OCV Intellectual Capital, LLC 2018-12-13 |
 ULTRABLADE 87596827 5477280 Live/Registered |
Wildfire, Inc. 2017-09-05 |
 ULTRABLADE 86548401 4830967 Live/Registered |
SPRINTURF INTELLECTUAL PROPERTY, LLC 2015-02-27 |
 ULTRABLADE 85633932 not registered Dead/Abandoned |
VALVOLINE LICENSING AND INTELLECTUAL PROPERTY LLC 2012-05-24 |
 ULTRABLADE 85006640 4168393 Dead/Cancelled |
OCV INTELLECTUAL CAPITAL, LLC 2010-04-05 |
 ULTRABLADE 79186650 not registered Live/Pending |
KACO new energy GmbH 2016-02-29 |
 ULTRABLADE 78878684 3357797 Dead/Cancelled |
Vertriebsgesellschaft Californian Products mbH 2006-05-08 |
 ULTRABLADE 78592109 3068082 Dead/Cancelled |
Specialty Surfaces International, Inc. 2005-03-22 |
 ULTRABLADE 76105213 2779943 Live/Registered |
FISKARS FINLAND OY AB 2000-08-08 |
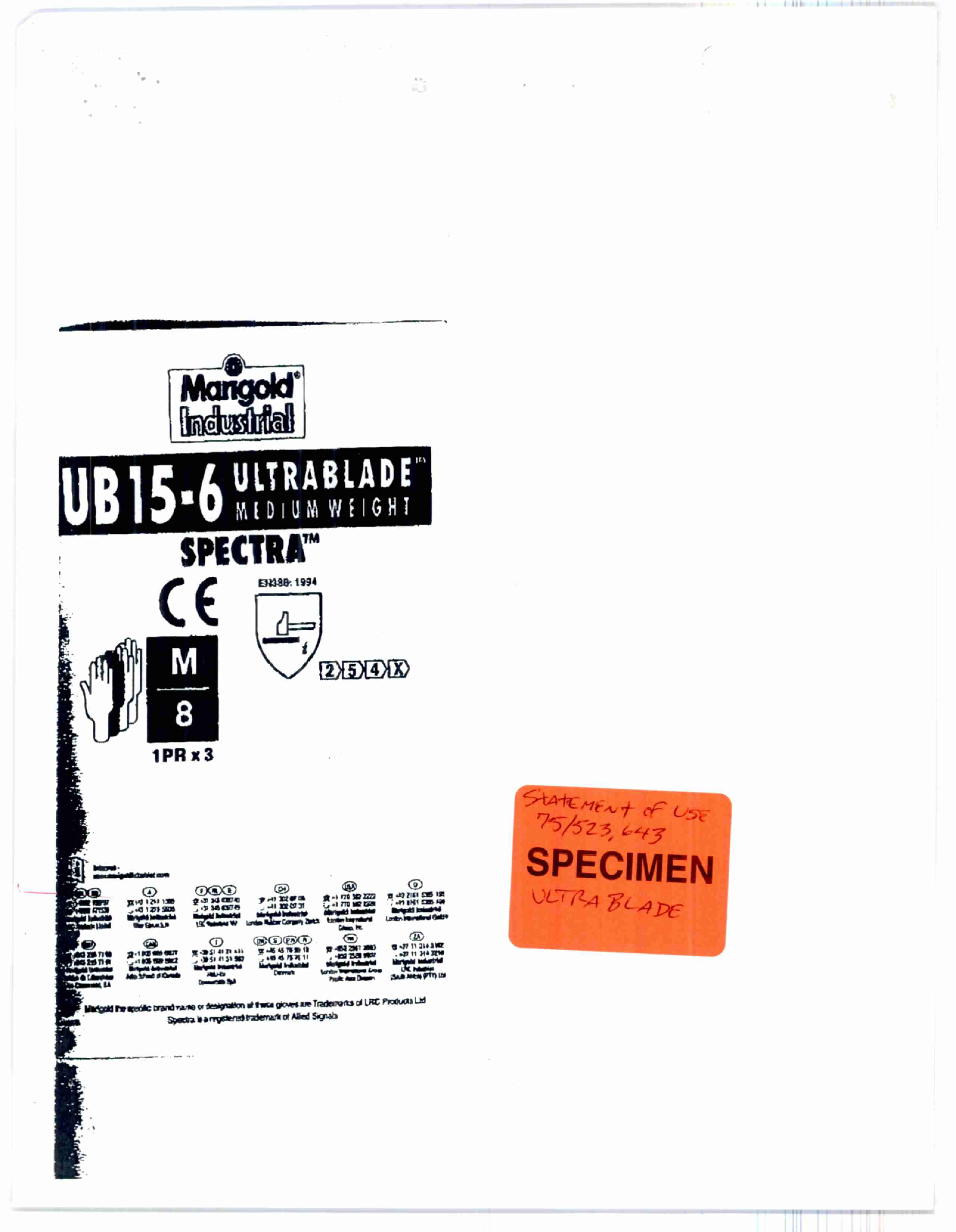 ULTRABLADE 75523643 2471944 Dead/Cancelled |
LRC PRODUCTS LIMITED 1998-07-22 |
 ULTRABLADE 75074103 2336953 Dead/Cancelled |
Vertriebsgesellschaft CALIFORNIAN PRODUCTS fur Sport- und Freizeitartikel mbH 1996-03-18 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.