ULTRAGOLD
Alloy, Gold-based Noble Metal
J.F. JELENKO & CO., INC.
The following data is part of a premarket notification filed by J.f. Jelenko & Co., Inc. with the FDA for Ultragold.
Pre-market Notification Details
| Device ID | K923817 |
| 510k Number | K923817 |
| Device Name: | ULTRAGOLD |
| Classification | Alloy, Gold-based Noble Metal |
| Applicant | J.F. JELENKO & CO., INC. 99 BUSINESS PARK DR. Armonk, NY 10504 |
| Contact | Costantino Volpe |
| Correspondent | Costantino Volpe J.F. JELENKO & CO., INC. 99 BUSINESS PARK DR. Armonk, NY 10504 |
| Product Code | EJT |
| CFR Regulation Number | 872.3060 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 1992-07-30 |
| Decision Date | 1993-03-11 |
Trademark Results [ULTRAGOLD]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ULTRAGOLD 97844649 not registered Live/Pending |
SoftWave Tissue Regeneration Technologies, LLC 2023-03-17 |
 ULTRAGOLD 88210140 not registered Live/Pending |
Agilent Technologies, Inc. 2018-11-29 |
 ULTRAGOLD 88015599 5759285 Live/Registered |
Standex International Corporation 2018-06-26 |
 ULTRAGOLD 85249720 4238359 Dead/Cancelled |
MILOR S.P.A. 2011-02-23 |
 ULTRAGOLD 78145719 not registered Dead/Abandoned |
JADO Hardware and Bathroom Manufacturing Corporation 2002-07-19 |
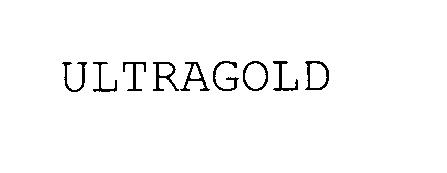 ULTRAGOLD 78025874 not registered Dead/Abandoned |
JADO Hardware and Bathroom Manufacturing Corporation 2000-09-14 |
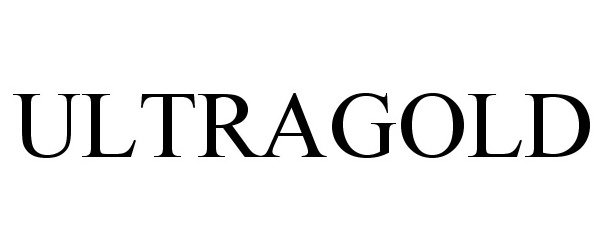 ULTRAGOLD 77368209 3566745 Live/Registered |
AGILENT TECHNOLOGIES, INC. 2008-01-10 |
 ULTRAGOLD 76298356 2538324 Dead/Cancelled |
UltraRF 2001-08-10 |
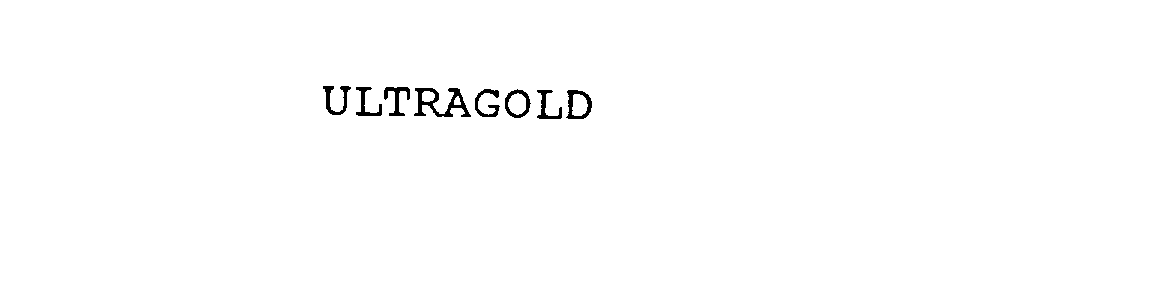 ULTRAGOLD 76079233 2537875 Live/Registered |
SURYS INC. 2000-06-26 |
 ULTRAGOLD 75168038 not registered Dead/Abandoned |
JADO BATHROOM AND HARDWARE MANUFACTURING CORPORATION 1996-09-18 |
 ULTRAGOLD 75168036 not registered Dead/Abandoned |
JADO BATHROOM AND HARDWARE MANUFACTURING CORPORATION 1996-09-18 |
 ULTRAGOLD 75168035 not registered Dead/Abandoned |
JADO BATHROOM AND HARDWARE MANUFACTURING CORPORATION 1996-09-18 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.