DRAPES
Drape, Surgical
PFB INTER-APPAREL CORP.
The following data is part of a premarket notification filed by Pfb Inter-apparel Corp. with the FDA for Drapes.
Pre-market Notification Details
| Device ID | K924335 |
| 510k Number | K924335 |
| Device Name: | DRAPES |
| Classification | Drape, Surgical |
| Applicant | PFB INTER-APPAREL CORP. 8751 N.W. 102ND ST. Medley, FL 33178 |
| Contact | Lemke Jr. |
| Correspondent | Lemke Jr. PFB INTER-APPAREL CORP. 8751 N.W. 102ND ST. Medley, FL 33178 |
| Product Code | KKX |
| CFR Regulation Number | 878.4370 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 1992-05-22 |
| Decision Date | 1993-09-07 |
Trademark Results [DRAPES]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
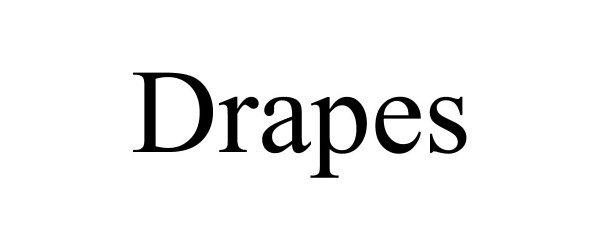 DRAPES 90506221 not registered Live/Pending |
Opfer, Eugene H. 2021-02-03 |
 DRAPES 90506221 not registered Live/Pending |
Opfer, Maile A. 2021-02-03 |
 DRAPES 73588225 1448599 Dead/Cancelled |
DRAPES SWIMWEAR, INC. 1986-03-17 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.