ECONOM
Speculum, Ent
RUDOLF RIESTER GMBH & CO. KG
The following data is part of a premarket notification filed by Rudolf Riester Gmbh & Co. Kg with the FDA for Econom.
Pre-market Notification Details
| Device ID | K925754 |
| 510k Number | K925754 |
| Device Name: | ECONOM |
| Classification | Speculum, Ent |
| Applicant | RUDOLF RIESTER GMBH & CO. KG POSTFACH 35 BRUCKSTRABE 31 D-72417 Jungingen, DE |
| Contact | Karlheinz Riester |
| Correspondent | Karlheinz Riester RUDOLF RIESTER GMBH & CO. KG POSTFACH 35 BRUCKSTRABE 31 D-72417 Jungingen, DE |
| Product Code | EPY |
| CFR Regulation Number | 878.1800 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 1992-11-13 |
| Decision Date | 1993-10-08 |
NIH GUDID Devices
| Device Identifier | submissionNumber | Supplement |
|---|---|---|
| 04045396130739 | K925754 | 000 |
| 04045396010291 | K925754 | 000 |
| 04045396010352 | K925754 | 000 |
| 04045396010369 | K925754 | 000 |
| 04045396010376 | K925754 | 000 |
| 04045396010383 | K925754 | 000 |
| 04045396010390 | K925754 | 000 |
| 04045396130593 | K925754 | 000 |
| 04045396130609 | K925754 | 000 |
| 04045396130616 | K925754 | 000 |
| 04045396002760 | K925754 | 000 |
Trademark Results [ECONOM]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ECONOM 86172138 4588644 Live/Registered |
Aesculap AG 2014-01-22 |
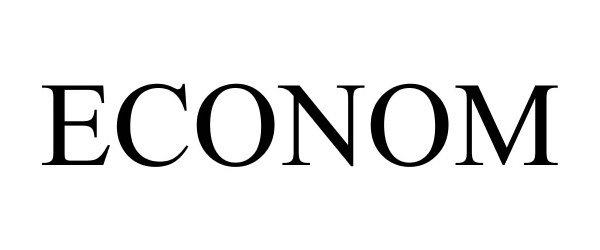 ECONOM 86172123 4588643 Live/Registered |
Aesculap AG 2014-01-22 |
 ECONOM 79315177 not registered Live/Pending |
Albert Kerbl GmbH 2021-06-09 |
 ECONOM 73763230 1585309 Dead/Cancelled |
ECONOMOS INDUSTRIAL PLASTICS, INC. 1988-11-14 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.