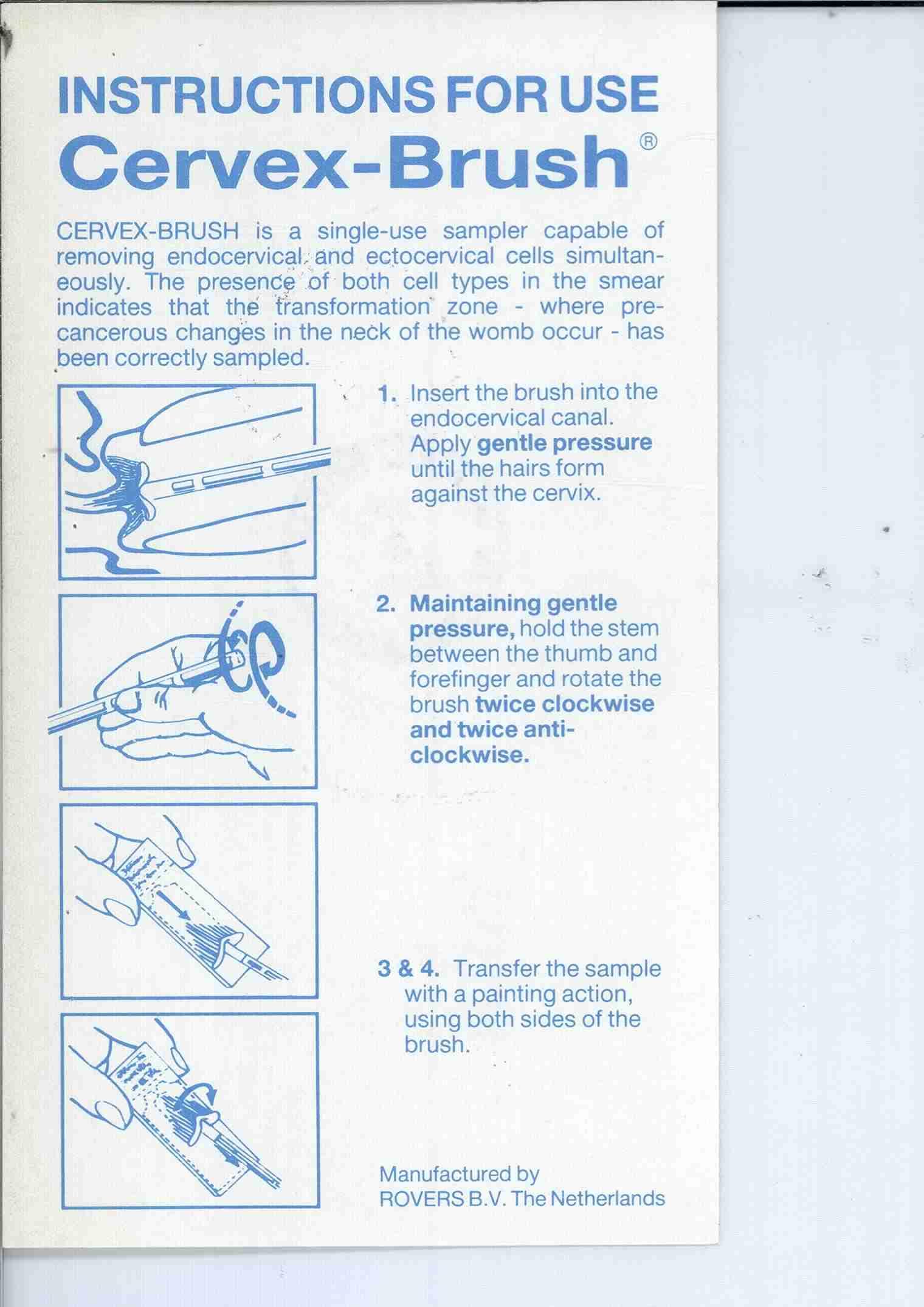
CERVEX-BRUSH
Spatula, Cervical, Cytological
ROVERS B.V.
The following data is part of a premarket notification filed by Rovers B.v. with the FDA for Cervex-brush.
Pre-market Notification Details
| Device ID | K930955 |
| 510k Number | K930955 |
| Device Name: | CERVEX-BRUSH |
| Classification | Spatula, Cervical, Cytological |
| Applicant | ROVERS B.V. 108 UNION ST. Rockaway, NJ 07866 |
| Contact | John Richardson |
| Correspondent | John Richardson ROVERS B.V. 108 UNION ST. Rockaway, NJ 07866 |
| Product Code | HHT |
| CFR Regulation Number | 884.4530 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 1993-02-23 |
| Decision Date | 1994-05-24 |
NIH GUDID Devices
| Device Identifier | submissionNumber | Supplement |
|---|---|---|
| 00613647000595 | K930955 | 000 |
| 28719189246395 | K930955 | 000 |
| 87191892466018 | K930955 | 000 |
| 08719189246056 | K930955 | 000 |
| 08719189246247 | K930955 | 000 |
| 08719189246506 | K930955 | 000 |
| 10382904905245 | K930955 | 000 |
| 87191892460016 | K930955 | 000 |
| 87191892460252 | K930955 | 000 |
| 87191892460566 | K930955 | 000 |
| 87191892462546 | K930955 | 000 |
| 00187207000290 | K930955 | 000 |
| 08719189246261 | K930955 | 000 |
Trademark Results [CERVEX-BRUSH]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 CERVEX-BRUSH 74154444 1855648 Live/Registered |
ROVERS MEDICAL DEVICES B.V. 1991-04-05 |
 CERVEX-BRUSH 74075103 not registered Dead/Abandoned |
Unimar, Inc. 1990-07-03 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.