HISTOFREEZER
Unit, Cryosurgical, Accessories
SOLARCARE TECHNOLOGIES CORP,INC.
The following data is part of a premarket notification filed by Solarcare Technologies Corp,inc. with the FDA for Histofreezer.
Pre-market Notification Details
| Device ID | K931299 |
| 510k Number | K931299 |
| Device Name: | HISTOFREEZER |
| Classification | Unit, Cryosurgical, Accessories |
| Applicant | SOLARCARE TECHNOLOGIES CORP,INC. 1745 EATON AVE. Bethlehem, PA 18018 -1799 |
| Contact | Sam Niedbala |
| Correspondent | Sam Niedbala SOLARCARE TECHNOLOGIES CORP,INC. 1745 EATON AVE. Bethlehem, PA 18018 -1799 |
| Product Code | GEH |
| CFR Regulation Number | 878.4350 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 1993-03-15 |
| Decision Date | 1993-08-11 |
Trademark Results [HISTOFREEZER]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
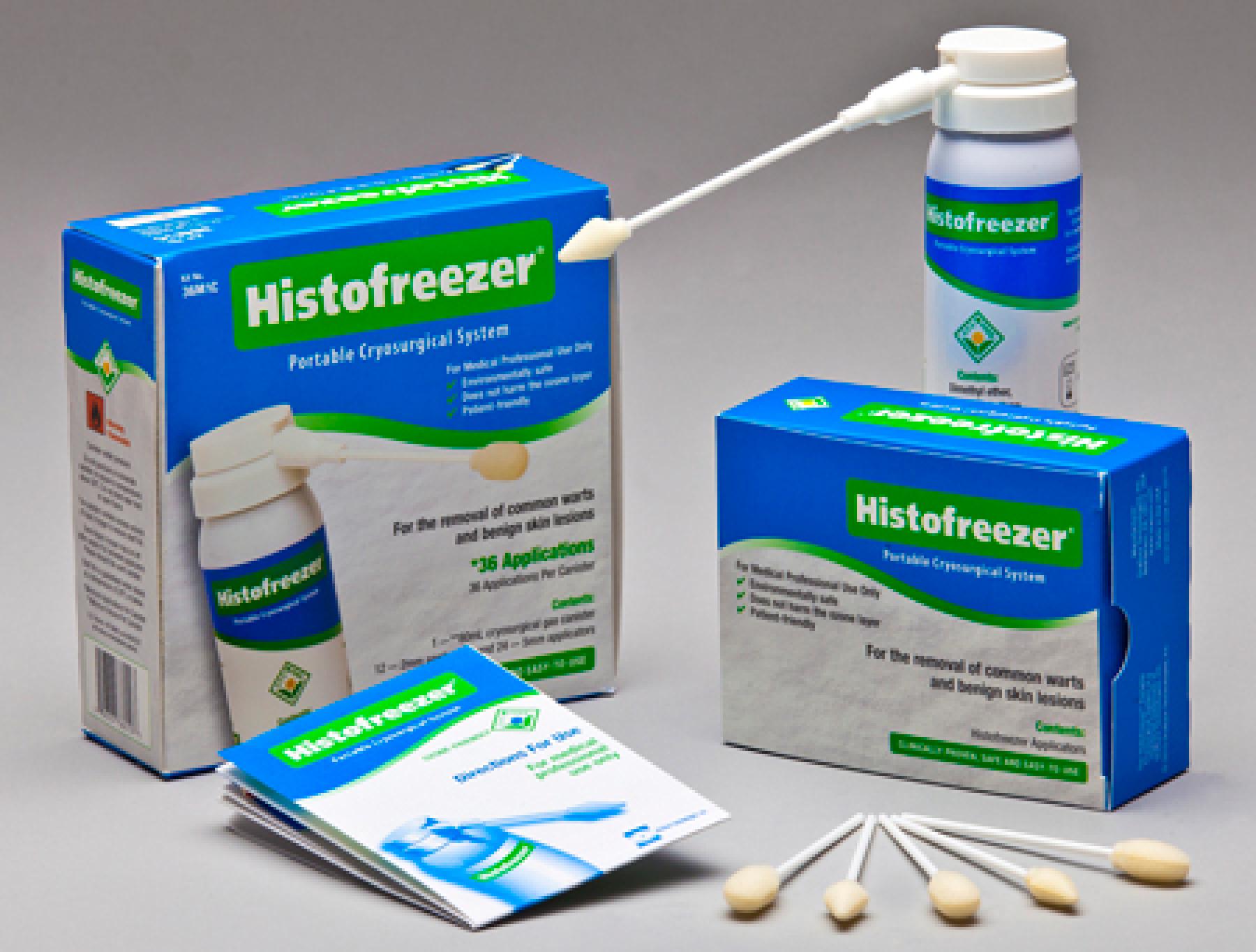 HISTOFREEZER 73741009 1525479 Live/Registered |
KONINKLIJKE UTERMOHLEN N.V. 1988-07-20 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.