BARRIER BONE WAX APPLICATOR
Orthopedic Manual Surgical Instrument
PHILLIPS PHARMACEUTICALS, INC.
The following data is part of a premarket notification filed by Phillips Pharmaceuticals, Inc. with the FDA for Barrier Bone Wax Applicator.
Pre-market Notification Details
| Device ID | K934260 |
| 510k Number | K934260 |
| Device Name: | BARRIER BONE WAX APPLICATOR |
| Classification | Orthopedic Manual Surgical Instrument |
| Applicant | PHILLIPS PHARMACEUTICALS, INC. 710 E. OGDEN AVE. SUITE 207 Naperville, IL 60563 |
| Contact | A.g. Phillips |
| Correspondent | A.g. Phillips PHILLIPS PHARMACEUTICALS, INC. 710 E. OGDEN AVE. SUITE 207 Naperville, IL 60563 |
| Product Code | LXH |
| CFR Regulation Number | 888.4540 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 1993-08-31 |
| Decision Date | 1994-01-26 |
Trademark Results [BARRIER BONE WAX APPLICATOR]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
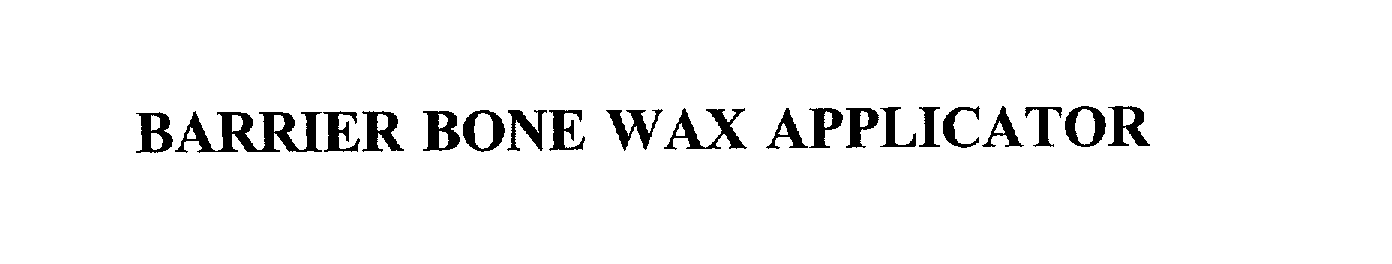 BARRIER BONE WAX APPLICATOR 75506610 2376571 Dead/Cancelled |
Phillips, Arnold G. 1998-06-22 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.