COLIBRI
Tube, Tracheal (w/wo Connector)
ICOR AB
The following data is part of a premarket notification filed by Icor Ab with the FDA for Colibri.
Pre-market Notification Details
| Device ID | K940041 |
| 510k Number | K940041 |
| Device Name: | COLIBRI |
| Classification | Tube, Tracheal (w/wo Connector) |
| Applicant | ICOR AB ULVSUNDAVAGEN 178 B Bromma, SE 161 30 |
| Contact | Andras Gedeon D.sc. |
| Correspondent | Andras Gedeon D.sc. ICOR AB ULVSUNDAVAGEN 178 B Bromma, SE 161 30 |
| Product Code | BTR |
| CFR Regulation Number | 868.5730 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 1994-01-04 |
| Decision Date | 1994-07-27 |
Trademark Results [COLIBRI]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 COLIBRI 98409062 not registered Live/Pending |
Daniel Garcia 2024-02-16 |
 COLIBRI 98409039 not registered Live/Pending |
Daniel Garcia 2024-02-16 |
 COLIBRI 98365972 not registered Live/Pending |
OLE LANGUAGE SCHOOLS, LLC 2024-01-19 |
 COLIBRI 98188732 not registered Live/Pending |
Decision Analyst, Inc. 2023-09-20 |
 COLIBRI 97851692 not registered Live/Pending |
Moov Furniture, LLC 2023-03-22 |
 COLIBRI 97524020 not registered Live/Pending |
Ridriguez, Blanca Olivia 2022-07-28 |
 COLIBRI 97091048 not registered Live/Pending |
McKissock, LLC 2021-10-25 |
 COLIBRI 90353881 not registered Live/Pending |
MANUFACTURAS 8-A, S.A. DE C.V. 2020-12-02 |
 COLIBRI 88884556 not registered Live/Pending |
ALPINE USA INC 2020-04-23 |
 COLIBRI 88578615 not registered Live/Pending |
The St. Barts Spirit Company 2019-08-14 |
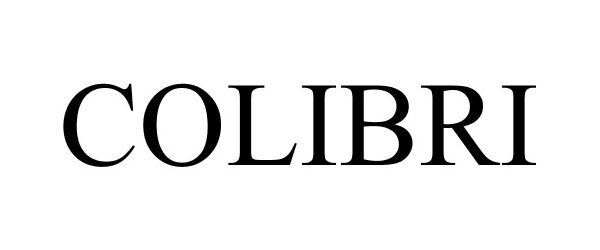 COLIBRI 88417322 not registered Live/Pending |
Jarritos, Inc. 2019-05-06 |
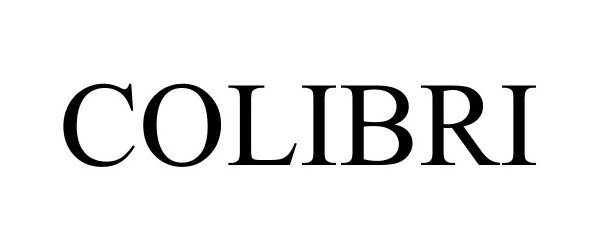 COLIBRI 88350739 not registered Live/Pending |
3NT MEDICAL LTD. 2019-03-21 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.