THE REEL THING
Catheter, Irrigation
BIO-MEDICAL DEVICES, INC.
The following data is part of a premarket notification filed by Bio-medical Devices, Inc. with the FDA for The Reel Thing.
Pre-market Notification Details
| Device ID | K940156 |
| 510k Number | K940156 |
| Device Name: | THE REEL THING |
| Classification | Catheter, Irrigation |
| Applicant | BIO-MEDICAL DEVICES, INC. 1752-A LANGLEY AVE. Irvine, CA 92614 |
| Contact | Nick Herbert |
| Correspondent | Nick Herbert BIO-MEDICAL DEVICES, INC. 1752-A LANGLEY AVE. Irvine, CA 92614 |
| Product Code | GBX |
| CFR Regulation Number | 878.4200 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 1994-01-12 |
| Decision Date | 1994-06-07 |
Trademark Results [THE REEL THING]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
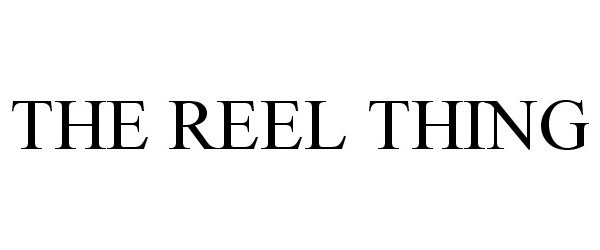 THE REEL THING 87113211 not registered Dead/Abandoned |
BALLY GAMING, INC. 2016-07-22 |
 THE REEL THING 81031579 1031579 Dead/Cancelled |
Reel Products, Inc. 0000-00-00 |
 THE REEL THING 76429472 not registered Dead/Abandoned |
Advance Magazine Publishers Inc. 2002-07-05 |
 THE REEL THING 76129490 2728558 Live/Registered |
The Reel Thing, Inc. 2000-09-15 |
 THE REEL THING 76083001 2605560 Dead/Cancelled |
Wirtshafter, Samuel 2000-07-05 |
 THE REEL THING 75019155 not registered Dead/Abandoned |
Ransomes America Corporation 1995-11-13 |
 THE REEL THING 74626171 not registered Dead/Abandoned |
Ransomes America Corporation 1995-01-26 |
 THE REEL THING 73720846 1509938 Live/Registered |
TULSA POWER PRODUCTS, INC. 1988-04-04 |
 THE REEL THING 73276037 1197010 Dead/Cancelled |
Reel Thing of California, Inc., The 1980-08-29 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.