ENDOSCOPE
Laparoscope, Gynecologic (and Accessories)
UNITED STATES SURGICAL, A DIVISION OF TYCO HEALTHC
The following data is part of a premarket notification filed by United States Surgical, A Division Of Tyco Healthc with the FDA for Endoscope.
Pre-market Notification Details
| Device ID | K944555 |
| 510k Number | K944555 |
| Device Name: | ENDOSCOPE |
| Classification | Laparoscope, Gynecologic (and Accessories) |
| Applicant | UNITED STATES SURGICAL, A DIVISION OF TYCO HEALTHC 150 GLOVER AVE. Norwalk, CT 06856 |
| Contact | Jonathan Gilbert |
| Correspondent | Jonathan Gilbert UNITED STATES SURGICAL, A DIVISION OF TYCO HEALTHC 150 GLOVER AVE. Norwalk, CT 06856 |
| Product Code | HET |
| CFR Regulation Number | 884.1720 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 1994-09-16 |
| Decision Date | 1994-12-30 |
Trademark Results [ENDOSCOPE]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
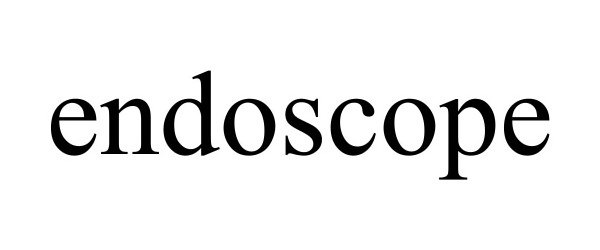 ENDOSCOPE 98127497 not registered Live/Pending |
CHEN, XIAOFENG 2023-08-10 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.