QUIK-STITCH
Laparoscope, General & Plastic Surgery
CORAL MEDICAL, INC.
The following data is part of a premarket notification filed by Coral Medical, Inc. with the FDA for Quik-stitch.
Pre-market Notification Details
| Device ID | K945170 |
| 510k Number | K945170 |
| Device Name: | QUIK-STITCH |
| Classification | Laparoscope, General & Plastic Surgery |
| Applicant | CORAL MEDICAL, INC. 5765 S. MONACO ST. Englewood, CO 80111 |
| Contact | Richard P Fleenor |
| Correspondent | Richard P Fleenor CORAL MEDICAL, INC. 5765 S. MONACO ST. Englewood, CO 80111 |
| Product Code | GCJ |
| CFR Regulation Number | 876.1500 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 1994-10-21 |
| Decision Date | 1995-01-18 |
Trademark Results [QUIK-STITCH]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
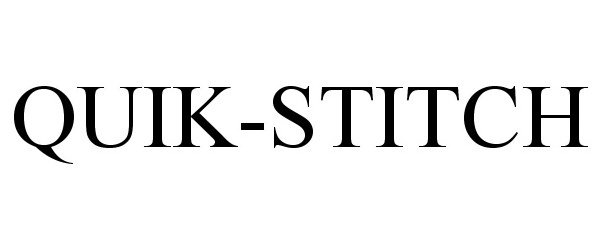 QUIK-STITCH 77633448 3647431 Dead/Cancelled |
PARE SURGICAL, INC. 2008-12-15 |
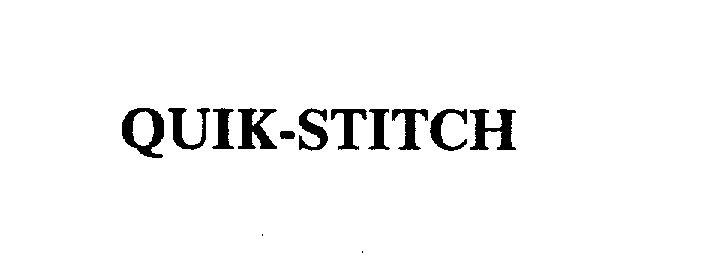 QUIK-STITCH 75160722 2300249 Dead/Cancelled |
Coral Medical, Inc. 1996-09-03 |
 QUIK-STITCH 73336075 not registered Dead/Abandoned |
TEXTURED YARN ARTS, INC. 1981-11-09 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.