ELMER
Powered Laser Surgical Instrument
BIOLOGICAL TECHNOLOGY CORP.
The following data is part of a premarket notification filed by Biological Technology Corp. with the FDA for Elmer.
Pre-market Notification Details
| Device ID | K952118 |
| 510k Number | K952118 |
| Device Name: | ELMER |
| Classification | Powered Laser Surgical Instrument |
| Applicant | BIOLOGICAL TECHNOLOGY CORP. 981 CALLE AMANECER Clemente, CA 92673 |
| Contact | Andrew Kimmel |
| Correspondent | Andrew Kimmel BIOLOGICAL TECHNOLOGY CORP. 981 CALLE AMANECER Clemente, CA 92673 |
| Product Code | GEX |
| CFR Regulation Number | 878.4810 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 1995-05-05 |
| Decision Date | 1995-08-07 |
Trademark Results [ELMER]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
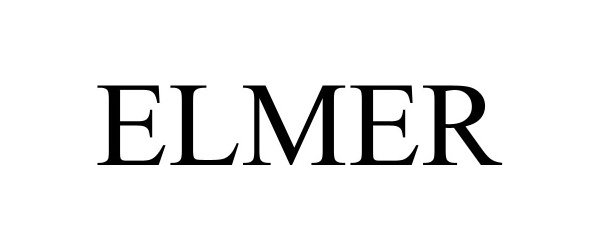 ELMER 98009557 not registered Live/Pending |
Montana Silversmiths, Inc. 2023-05-23 |
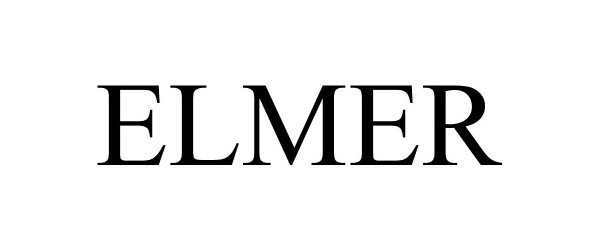 ELMER 87603715 5840258 Live/Registered |
Sperry Rail Service, Inc. 2017-09-11 |
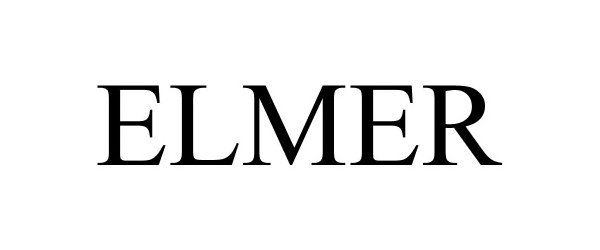 ELMER 85349283 4192985 Dead/Cancelled |
Montana Silversmiths, Inc. 2011-06-17 |
 ELMER 79238736 not registered Live/Pending |
L.T. - AQUA + 2018-02-12 |
 ELMER 79238020 not registered Live/Pending |
L.T. - AQUA + 2018-02-12 |
 ELMER 75189497 not registered Dead/Abandoned |
Elmer's Investments, Inc. 1996-10-29 |
 ELMER 72242579 0834047 Dead/Expired |
ELMAG 1966-04-04 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.