RETRAC
Material, Impression
CENTRIX, INC.
The following data is part of a premarket notification filed by Centrix, Inc. with the FDA for Retrac.
Pre-market Notification Details
| Device ID | K952728 |
| 510k Number | K952728 |
| Device Name: | RETRAC |
| Classification | Material, Impression |
| Applicant | CENTRIX, INC. 770 RIVER RD. Shelton, CT 06484 -5458 |
| Contact | John Discko |
| Correspondent | John Discko CENTRIX, INC. 770 RIVER RD. Shelton, CT 06484 -5458 |
| Product Code | ELW |
| CFR Regulation Number | 872.3660 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 1995-06-14 |
| Decision Date | 1995-09-29 |
NIH GUDID Devices
| Device Identifier | submissionNumber | Supplement |
|---|---|---|
| 00817051021270 | K952728 | 000 |
| 00817051021263 | K952728 | 000 |
| 00817051021256 | K952728 | 000 |
| 00817051021249 | K952728 | 000 |
| 00817051021232 | K952728 | 000 |
| 00817051021225 | K952728 | 000 |
Trademark Results [RETRAC]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 RETRAC 86850343 5177480 Live/Registered |
Petersen, Carter 2015-12-15 |
 RETRAC 86725012 4954783 Live/Registered |
CURT MANUFACTURING, LLC 2015-08-14 |
 RETRAC 78721066 not registered Dead/Abandoned |
AstraZeneca AB 2005-09-27 |
 RETRAC 78457320 3081755 Dead/Cancelled |
ARROW ELECTRONICS, INC. 2004-07-27 |
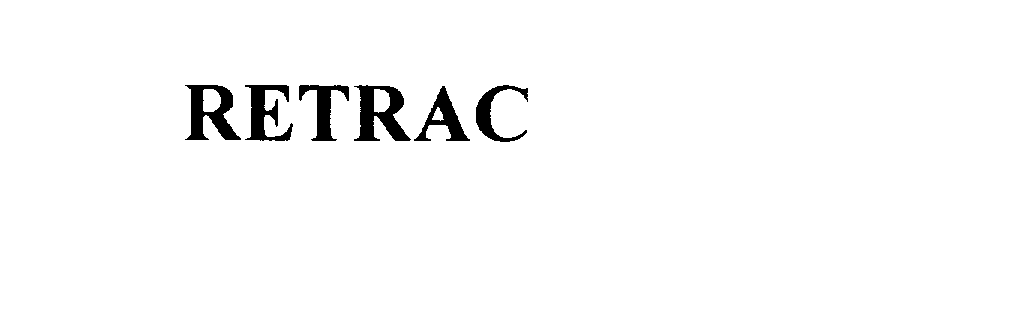 RETRAC 76059320 not registered Dead/Abandoned |
Retrack USA, Inc. 2000-05-30 |
 RETRAC 73166340 1129798 Dead/Cancelled |
WILHELM HEGENSCHEIDT GMBH 1978-04-14 |
 RETRAC 71420216 0371994 Dead/Cancelled |
CARTER BROTHERS, INCORPORATED 1939-06-06 |
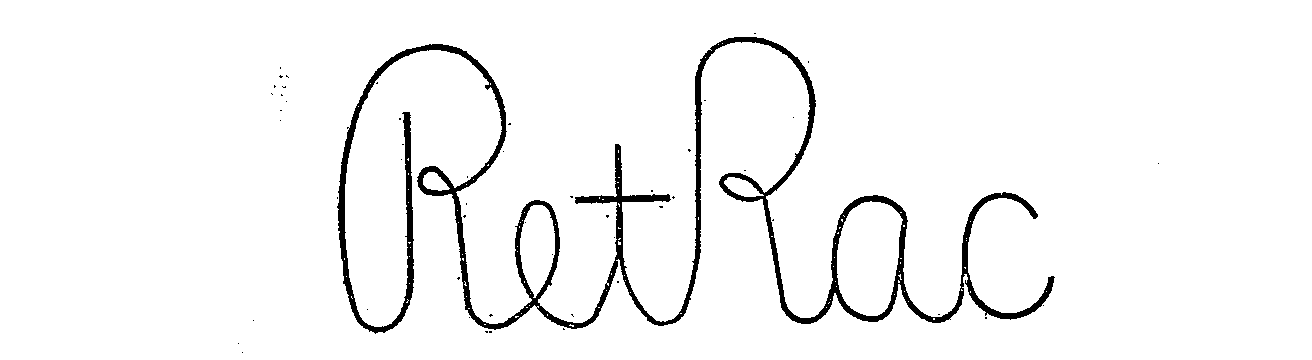 RETRAC 71308574 0282411 Dead/Expired |
CARTER BROTHERS, INCORPORATED 1930-12-04 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.