E-Z FLEX
Exerciser, Powered
FLUID MOTION BIOTECHNOLOGIES, INC.
The following data is part of a premarket notification filed by Fluid Motion Biotechnologies, Inc. with the FDA for E-z Flex.
Pre-market Notification Details
| Device ID | K953456 |
| 510k Number | K953456 |
| Device Name: | E-Z FLEX |
| Classification | Exerciser, Powered |
| Applicant | FLUID MOTION BIOTECHNOLOGIES, INC. 55 NORTHER BLVD. SUITE 410 Great Neck, NY 11021 |
| Contact | Susan D Goldstein-falk |
| Correspondent | Susan D Goldstein-falk FLUID MOTION BIOTECHNOLOGIES, INC. 55 NORTHER BLVD. SUITE 410 Great Neck, NY 11021 |
| Product Code | BXB |
| CFR Regulation Number | 890.5380 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 1995-07-19 |
| Decision Date | 1996-01-26 |
Trademark Results [E-Z FLEX]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
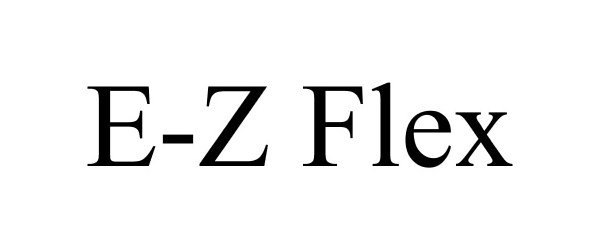 E-Z FLEX 87106595 5212307 Live/Registered |
Garcoa Inc. 2016-07-17 |
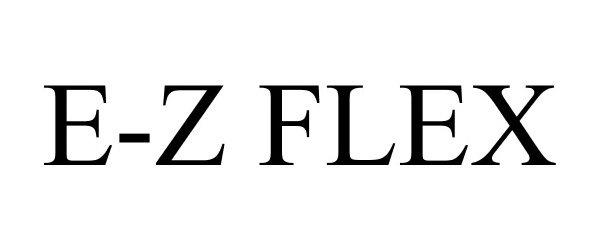 E-Z FLEX 77533429 3709942 Live/Registered |
Dexter Axle Company 2008-07-29 |
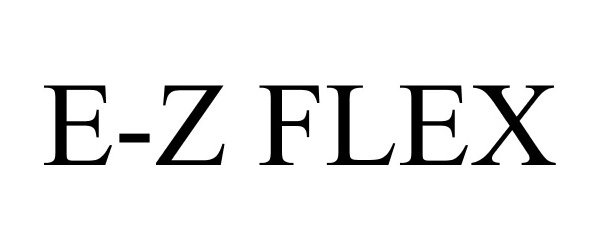 E-Z FLEX 77048868 not registered Dead/Abandoned |
Dexter Axle Company 2006-11-21 |
 E-Z FLEX 74710713 2077977 Dead/Cancelled |
ATOS MEDICAL AB 1995-08-03 |
 E-Z FLEX 73541710 1376929 Dead/Cancelled |
DAYCO CORPORATION 1985-06-06 |
 E-Z FLEX 73506881 1366672 Dead/Cancelled |
DAYCO PRODUCTS, INC. 1984-11-02 |
 E-Z FLEX 73506881 1366672 Dead/Cancelled |
DAYCO CORPORATION 1984-11-02 |
 E-Z FLEX 72447888 0983094 Live/Registered |
NORTON COMPANY 1973-02-05 |
 E-Z FLEX 72447833 0987778 Dead/Cancelled |
DAYCO CORPORATION 1973-02-05 |
 E-Z FLEX 72103403 0716952 Dead/Expired |
CLIFFORD W. GUSTAFSON 1960-08-25 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.