STREP A OIA MAX
Antisera, All Groups, Streptococcus Spp.
BIOSTAR, INC.
The following data is part of a premarket notification filed by Biostar, Inc. with the FDA for Strep A Oia Max.
Pre-market Notification Details
| Device ID | K962060 |
| 510k Number | K962060 |
| Device Name: | STREP A OIA MAX |
| Classification | Antisera, All Groups, Streptococcus Spp. |
| Applicant | BIOSTAR, INC. 6655 LOOKOUT RD. Boulder, CO 80301 |
| Contact | Lyndal K Hesterberg |
| Correspondent | Lyndal K Hesterberg BIOSTAR, INC. 6655 LOOKOUT RD. Boulder, CO 80301 |
| Product Code | GTZ |
| CFR Regulation Number | 866.3740 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 1996-05-28 |
| Decision Date | 1997-07-23 |
Trademark Results [STREP A OIA MAX]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
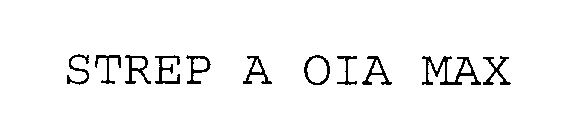 STREP A OIA MAX 76253162 2911935 Dead/Cancelled |
INVERNESS MEDICAL - BIOSTAR INC. INVERNESS MEDICAL PROFESSIONAL DIAGNOSTICS 2001-05-04 |
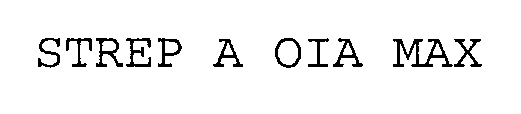 STREP A OIA MAX 76252224 2720521 Dead/Cancelled |
INVERNESS MEDICAL - BIOSTAR INC. INVERNESS MEDICAL PROFESSIONAL DIAGNOSTICS 2001-05-04 |
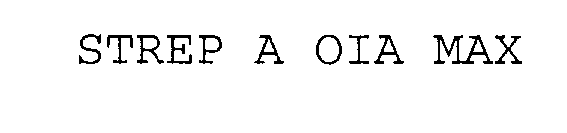 STREP A OIA MAX 76251323 2950789 Dead/Cancelled |
INVERNESS MEDICAL - BIOSTAR INC. INVERNESS MEDICAL PROFESSIONAL DIAGNOSTICS 2001-05-04 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.