AUTOPRIME
Filter, Infusion Line
ARBOR TECHNOLOGIES, INC.
The following data is part of a premarket notification filed by Arbor Technologies, Inc. with the FDA for Autoprime.
Pre-market Notification Details
| Device ID | K964283 |
| 510k Number | K964283 |
| Device Name: | AUTOPRIME |
| Classification | Filter, Infusion Line |
| Applicant | ARBOR TECHNOLOGIES, INC. 401 WEST MORGAN RD. Ann Arbor, MI 48108 |
| Contact | Dawn I Moore |
| Correspondent | Dawn I Moore ARBOR TECHNOLOGIES, INC. 401 WEST MORGAN RD. Ann Arbor, MI 48108 |
| Product Code | FPB |
| CFR Regulation Number | 880.5440 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 1996-10-28 |
| Decision Date | 1997-01-22 |
| Summary: | summary |
Trademark Results [AUTOPRIME]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 AUTOPRIME 98224313 not registered Live/Pending |
Shanghai mobibot technology co,. Ltd. 2023-10-14 |
 AUTOPRIME 98224312 not registered Live/Pending |
Shanghai mobibot technology co,. Ltd. 2023-10-14 |
 AUTOPRIME 98224309 not registered Live/Pending |
Shanghai mobibot technology co,. Ltd. 2023-10-14 |
 AUTOPRIME 98224308 not registered Live/Pending |
Shanghai mobibot technology co,. Ltd. 2023-10-14 |
 AUTOPRIME 98219932 not registered Live/Pending |
Shanghai mobibot technology co,. Ltd. 2023-10-11 |
 AUTOPRIME 98164669 not registered Live/Pending |
Designetics, Inc. 2023-09-05 |
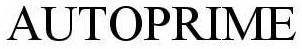 AUTOPRIME 78512321 3335983 Dead/Cancelled |
SPP Pumps Limited 2004-11-05 |
 AUTOPRIME 75224872 2181908 Dead/Cancelled |
AutoPrime, Inc. 1997-01-13 |
 AUTOPRIME 74598730 1931524 Live/Registered |
PPG INDUSTRIES OHIO, INC. 1994-11-14 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.