CLASSIC MATCH
Sunglasses (non-prescription Including Photosensitive)
DELAGAR DIVISION BELCAM, INC.
The following data is part of a premarket notification filed by Delagar Division Belcam, Inc. with the FDA for Classic Match.
Pre-market Notification Details
| Device ID | K971104 |
| 510k Number | K971104 |
| Device Name: | CLASSIC MATCH |
| Classification | Sunglasses (non-prescription Including Photosensitive) |
| Applicant | DELAGAR DIVISION BELCAM, INC. 27 MONTGOMERY ST. Rouses Point, NY 12979 -0277 |
| Contact | Cally Lunetta |
| Correspondent | Cally Lunetta DELAGAR DIVISION BELCAM, INC. 27 MONTGOMERY ST. Rouses Point, NY 12979 -0277 |
| Product Code | HQY |
| CFR Regulation Number | 886.5850 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 1997-03-26 |
| Decision Date | 1997-05-06 |
Trademark Results [CLASSIC MATCH]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
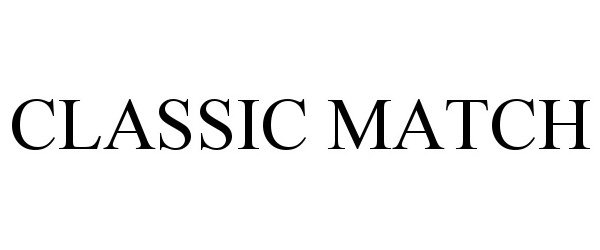 CLASSIC MATCH 97269703 not registered Live/Pending |
Belcam Inc. 2022-02-16 |
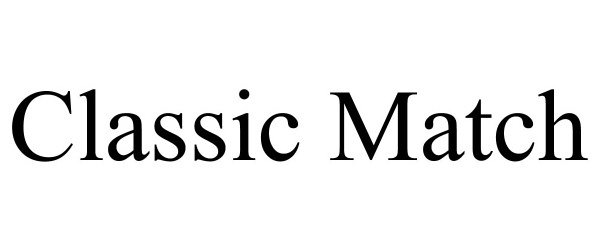 CLASSIC MATCH 88804364 not registered Live/Pending |
Specialty Cartridge, Inc. 2020-02-20 |
 CLASSIC MATCH 73639685 1454820 Live/Registered |
BELCAM INC. 1987-01-14 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.