ARAGO
Powered Laser Surgical Instrument
PREMIER LASER SYSTEMS, INC.
The following data is part of a premarket notification filed by Premier Laser Systems, Inc. with the FDA for Arago.
Pre-market Notification Details
| Device ID | K971118 |
| 510k Number | K971118 |
| Device Name: | ARAGO |
| Classification | Powered Laser Surgical Instrument |
| Applicant | PREMIER LASER SYSTEMS, INC. 3 MORGAN Irvine, CA 92718 |
| Contact | Lisa Mojica |
| Correspondent | Lisa Mojica PREMIER LASER SYSTEMS, INC. 3 MORGAN Irvine, CA 92718 |
| Product Code | GEX |
| CFR Regulation Number | 878.4810 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 1997-03-21 |
| Decision Date | 1997-12-16 |
Trademark Results [ARAGO]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ARAGO 97575229 not registered Live/Pending |
MEDIAKEYS GROUP 2022-09-01 |
 ARAGO 97369689 not registered Live/Pending |
C & D OnSite 2022-04-19 |
 ARAGO 87569525 5396507 Live/Registered |
arago GmbH 2017-08-15 |
 ARAGO 87534349 not registered Live/Pending |
arago GmbH 2017-07-19 |
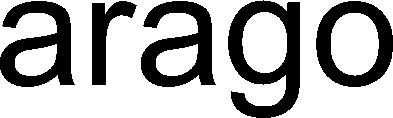 ARAGO 79231781 5671990 Live/Registered |
nora systems GmbH 2017-12-21 |
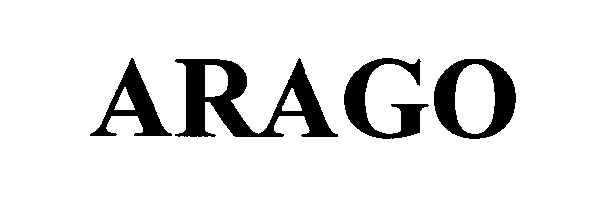 ARAGO 76625906 3026273 Live/Registered |
Smithsonian Institution 2004-12-29 |
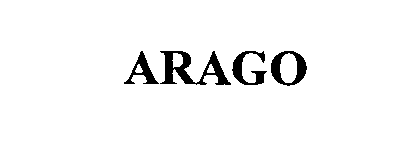 ARAGO 76468882 not registered Dead/Abandoned |
SNAP-ON INCORPORATED 2002-11-21 |
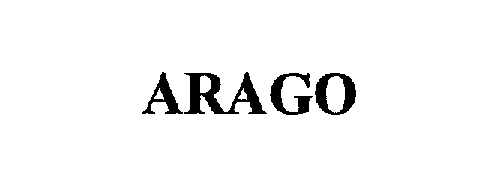 ARAGO 75307008 2362621 Dead/Cancelled |
Premier Laser Systems, Inc. 1997-06-11 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.