SNOREX
Device, Jaw Repositioning
SNOREX (NZ) LTD.
The following data is part of a premarket notification filed by Snorex (nz) Ltd. with the FDA for Snorex.
Pre-market Notification Details
| Device ID | K971818 |
| 510k Number | K971818 |
| Device Name: | SNOREX |
| Classification | Device, Jaw Repositioning |
| Applicant | SNOREX (NZ) LTD. 8210 CARRLEIGH PKWY. Springfield, VA 22152 |
| Contact | Vernon Pribble |
| Correspondent | Vernon Pribble SNOREX (NZ) LTD. 8210 CARRLEIGH PKWY. Springfield, VA 22152 |
| Product Code | LQZ |
| CFR Regulation Number | 872.5570 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 1997-05-16 |
| Decision Date | 1997-12-18 |
| Summary: | summary |
Trademark Results [SNOREX]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 SNOREX 98643795 not registered Live/Pending |
MARC J KAYEM MD INC 2024-07-11 |
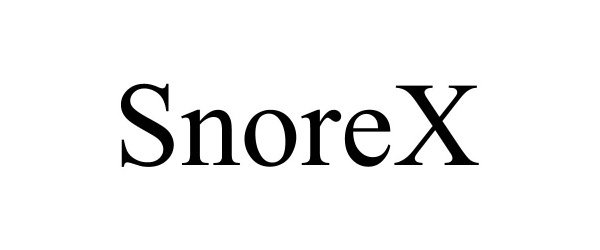 SNOREX 97092055 not registered Live/Pending |
MARC J KAYEM MD INC 2021-10-26 |
 SNOREX 75159035 2160922 Dead/Cancelled |
SELECT NET MARKETING, INC. 1996-08-30 |
 SNOREX 74257313 not registered Dead/Abandoned |
SOMTEK, INC. 1992-03-20 |
 SNOREX 73597200 not registered Dead/Abandoned |
C.H.S. MARKETING, INC. 1986-05-06 |
 SNOREX 73591148 not registered Dead/Abandoned |
UNITED-GUARDIAN, INC. 1986-04-01 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.