VAPR
Electrosurgical, Cutting & Coagulation & Accessories
MITEK PRODUCTS
The following data is part of a premarket notification filed by Mitek Products with the FDA for Vapr.
Pre-market Notification Details
| Device ID | K974022 |
| 510k Number | K974022 |
| Device Name: | VAPR |
| Classification | Electrosurgical, Cutting & Coagulation & Accessories |
| Applicant | MITEK PRODUCTS 60 GLACIER DR. Westwood, MA 02090 |
| Contact | Edward F Kent |
| Correspondent | Edward F Kent MITEK PRODUCTS 60 GLACIER DR. Westwood, MA 02090 |
| Product Code | GEI |
| CFR Regulation Number | 878.4400 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 1997-10-14 |
| Decision Date | 1998-01-12 |
| Summary: | summary |
Trademark Results [VAPR]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 VAPR 98424618 not registered Live/Pending |
MS Sedco, Inc. 2024-02-28 |
 VAPR 98395390 not registered Live/Pending |
Capstan Ag Systems, Inc. 2024-02-07 |
 VAPR 97563876 not registered Live/Pending |
Nice Recovery Systems LLC 2022-08-25 |
 VAPR 97066025 not registered Live/Pending |
Industrial Chemicals LLC 2021-10-08 |
 VAPR 97065999 not registered Live/Pending |
Industrial Chemicals LLC 2021-10-08 |
 VAPR 88637942 not registered Live/Pending |
GEODynamics, Inc. 2019-10-01 |
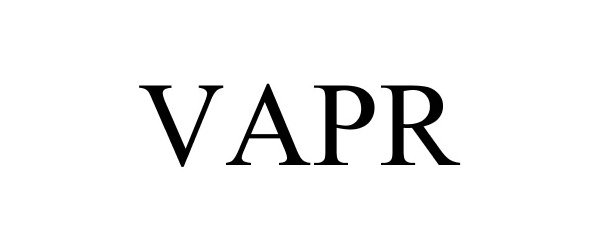 VAPR 88319705 not registered Live/Pending |
National Oilwell Varco, L.P. 2019-02-28 |
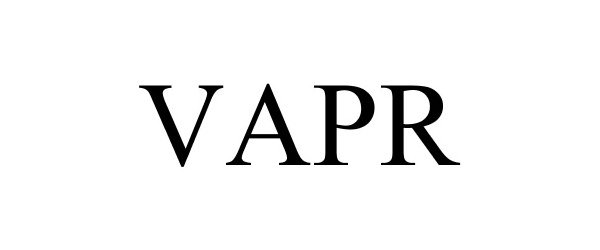 VAPR 87077624 not registered Dead/Abandoned |
Vaprwear, Gear, LLC 2016-06-20 |
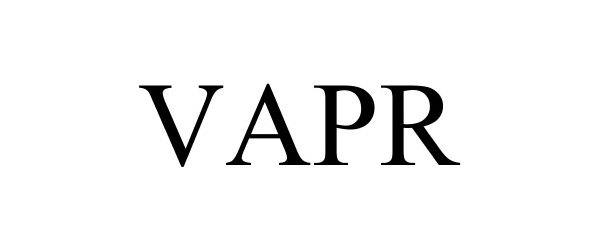 VAPR 86245969 not registered Dead/Abandoned |
Henderson Aquatics, Inc. 2014-04-08 |
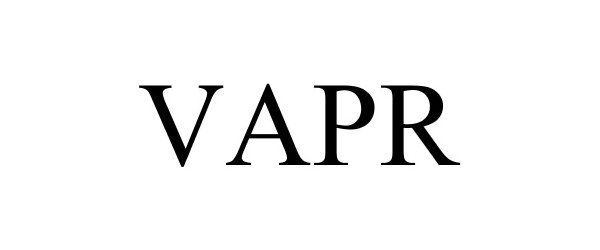 VAPR 77811937 not registered Dead/Abandoned |
Remington Health Products, LLC 2009-08-25 |
 VAPR 75437271 2295182 Live/Registered |
JOHNSON & JOHNSON 1998-02-19 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.