IMAGEQUEST
System, Digital Image Communications, Radiological
VISUAL MED, INC.
The following data is part of a premarket notification filed by Visual Med, Inc. with the FDA for Imagequest.
Pre-market Notification Details
| Device ID | K974707 |
| 510k Number | K974707 |
| Device Name: | IMAGEQUEST |
| Classification | System, Digital Image Communications, Radiological |
| Applicant | VISUAL MED, INC. 3695 CENTRE CIRCLE DR. Ft. Mill, SC 29715 |
| Product Code | LMD |
| CFR Regulation Number | 892.2020 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 1997-12-16 |
| Decision Date | 1998-07-31 |
Trademark Results [IMAGEQUEST]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
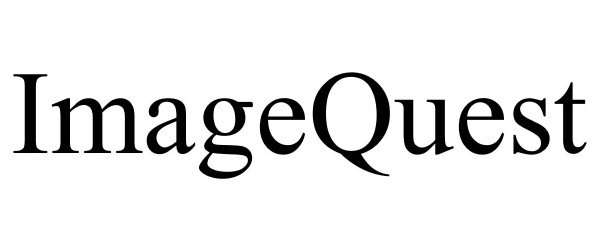 IMAGEQUEST 86490224 4912175 Live/Registered |
Encyclopaedia Britannica, Inc. 2014-12-24 |
 IMAGEQUEST 85655609 4291676 Live/Registered |
Florida Business Technologies, LLC 2012-06-19 |
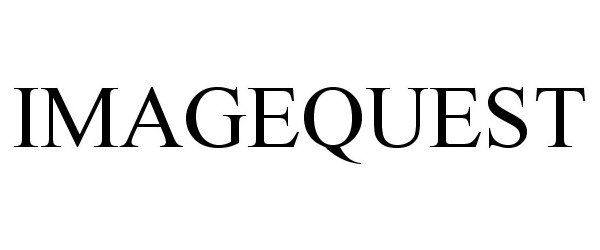 IMAGEQUEST 77076168 3550719 Dead/Cancelled |
Thermo Fisher Scientific Inc. 2007-01-04 |
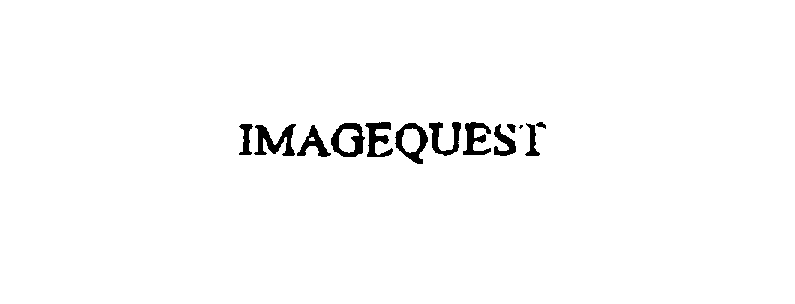 IMAGEQUEST 76116265 not registered Dead/Abandoned |
Auto F/X Corporation 2000-08-24 |
 IMAGEQUEST 75812112 not registered Dead/Abandoned |
Auto F/x Coqorarion 1999-09-30 |
 IMAGEQUEST 75581729 2451526 Live/Registered |
IMAGEQUEST CO., LTD. 1998-11-02 |
 IMAGEQUEST 75456728 2390261 Dead/Cancelled |
GROUP360, INC. 1998-03-26 |
 IMAGEQUEST 74237105 not registered Dead/Abandoned |
Hamamatsu Photonics K.K. 1992-01-13 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.