EQUINOXE
Bath, Hydro-massage
BOUVIER HYDRO, INC.
The following data is part of a premarket notification filed by Bouvier Hydro, Inc. with the FDA for Equinoxe.
Pre-market Notification Details
| Device ID | K980920 |
| 510k Number | K980920 |
| Device Name: | EQUINOXE |
| Classification | Bath, Hydro-massage |
| Applicant | BOUVIER HYDRO, INC. 100 WALNUT ST. SUITE 20 P.O. BOX 2874 Champlain,, NY 12919 |
| Contact | Alain Jouan |
| Correspondent | Alain Jouan BOUVIER HYDRO, INC. 100 WALNUT ST. SUITE 20 P.O. BOX 2874 Champlain,, NY 12919 |
| Product Code | ILJ |
| CFR Regulation Number | 890.5100 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 1998-03-10 |
| Decision Date | 1998-07-16 |
Trademark Results [EQUINOXE]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 EQUINOXE 76533714 3121160 Live/Registered |
Exactech, Inc. 2003-07-31 |
 EQUINOXE 74669086 2299128 Dead/Cancelled |
Caran d'Ache SA 1995-05-02 |
 EQUINOXE 74551690 2021609 Dead/Cancelled |
EQUINOXE 1994-07-20 |
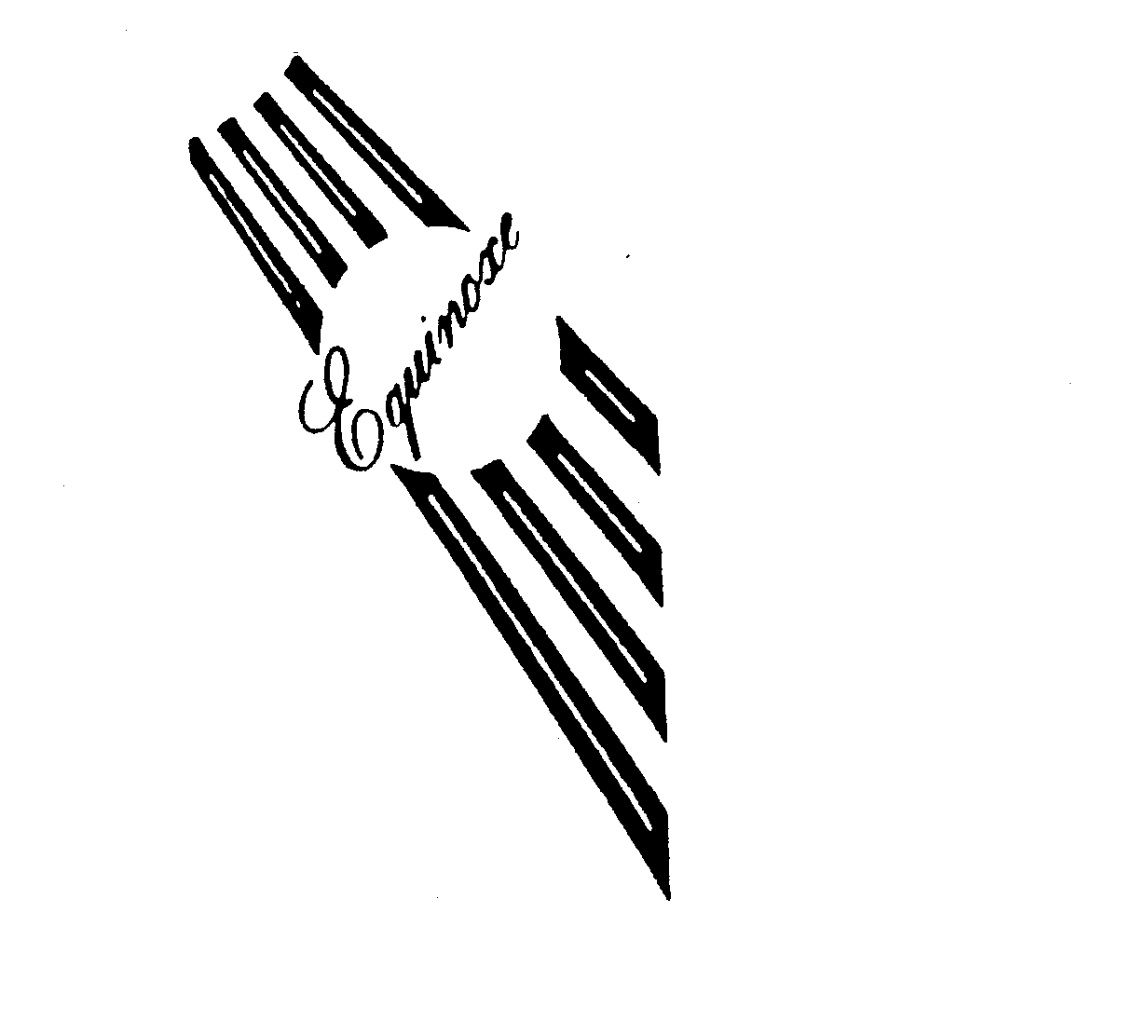 EQUINOXE 74284084 1822644 Dead/Cancelled |
C.M.S. MUSIC INC. 1992-06-12 |
 EQUINOXE 74283205 1831282 Dead/Cancelled |
C.M.S. MUSIC INC. 1992-06-09 |
 EQUINOXE 73828780 1606586 Dead/Cancelled |
ETABLISSEMENTS DE LOISY & GELET 1989-10-02 |
 EQUINOXE 73734775 1550376 Live/Registered |
ETABLISSEMENTS DE LOISY & GELET 1988-06-17 |
 EQUINOXE 73716743 1535166 Dead/Cancelled |
ALLIA 1988-03-14 |
 EQUINOXE 73285261 1248395 Dead/Cancelled |
Lainiere de Roubaix 1980-11-10 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.