SHEER GLYDE DAMS
Barrier, Std, Oral Sex
LOUISE C. MYERS
The following data is part of a premarket notification filed by Louise C. Myers with the FDA for Sheer Glyde Dams.
Pre-market Notification Details
| Device ID | K990067 |
| 510k Number | K990067 |
| Device Name: | SHEER GLYDE DAMS |
| Classification | Barrier, Std, Oral Sex |
| Applicant | LOUISE C. MYERS 14808 N.E. 66TH ST. Redmond, WA 98052 -4712 |
| Contact | Louise C Myers |
| Correspondent | Louise C Myers LOUISE C. MYERS 14808 N.E. 66TH ST. Redmond, WA 98052 -4712 |
| Product Code | MSC |
| CFR Regulation Number | 884.5300 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 1999-01-08 |
| Decision Date | 1999-02-19 |
| Summary: | summary |
NIH GUDID Devices
| Device Identifier | submissionNumber | Supplement |
|---|---|---|
| 80726893115107 | K990067 | 000 |
| 80726893114919 | K990067 | 000 |
Trademark Results [SHEER GLYDE DAMS]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
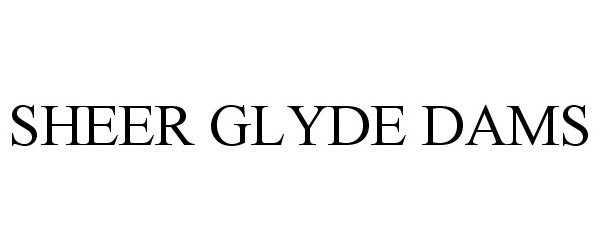 SHEER GLYDE DAMS 86786583 not registered Dead/Abandoned |
Global Protection Corp. 2015-10-13 |
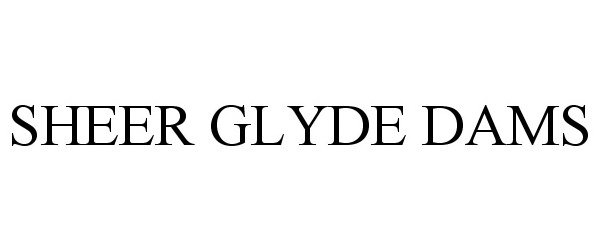 SHEER GLYDE DAMS 86783822 5234719 Live/Registered |
SOOKA INC. 2015-10-09 |
 SHEER GLYDE DAMS 75508818 2274241 Dead/Cancelled |
GLYDE USA, INC. 1998-06-25 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.