TWILIGHT
Powered Laser Surgical Instrument
BIOLASE TECHNOLOGY, INC.
The following data is part of a premarket notification filed by Biolase Technology, Inc. with the FDA for Twilight.
Pre-market Notification Details
| Device ID | K991994 |
| 510k Number | K991994 |
| Device Name: | TWILIGHT |
| Classification | Powered Laser Surgical Instrument |
| Applicant | BIOLASE TECHNOLOGY, INC. 981 CALLE AMANECER San Clemente, CA 92673 |
| Contact | Ioana M Rizoiu |
| Correspondent | Ioana M Rizoiu BIOLASE TECHNOLOGY, INC. 981 CALLE AMANECER San Clemente, CA 92673 |
| Product Code | GEX |
| CFR Regulation Number | 878.4810 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 1999-06-14 |
| Decision Date | 1999-09-10 |
| Summary: | summary |
Trademark Results [TWILIGHT]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 TWILIGHT 98738104 not registered Live/Pending |
SDIP LLC 2024-09-06 |
 TWILIGHT 98619950 not registered Live/Pending |
VISIOLITE LLC 2024-06-26 |
 TWILIGHT 98388687 not registered Live/Pending |
NOBY INC 2024-02-02 |
 TWILIGHT 98388687 not registered Live/Pending |
Noah Doris 2024-02-02 |
 TWILIGHT 98388687 not registered Live/Pending |
BYRON D'MELLO 2024-02-02 |
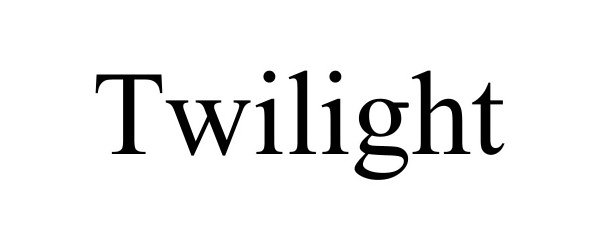 TWILIGHT 98282663 not registered Live/Pending |
At Twilight Hospice & Palliative Care LLC 2023-11-22 |
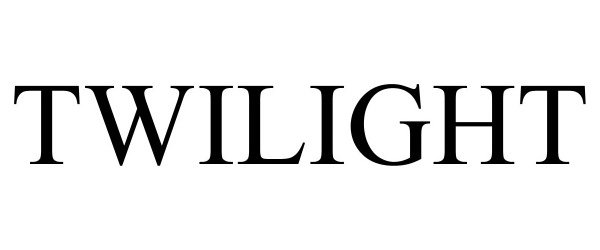 TWILIGHT 98170517 not registered Live/Pending |
Sanniti L.L.C. 2023-09-08 |
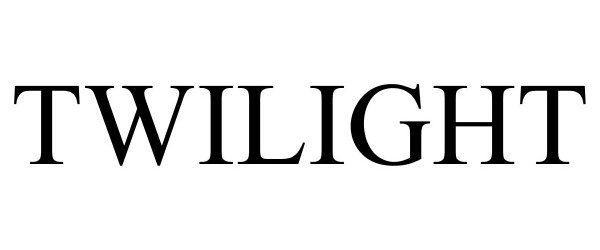 TWILIGHT 98067535 not registered Live/Pending |
Knight, JZ 2023-06-30 |
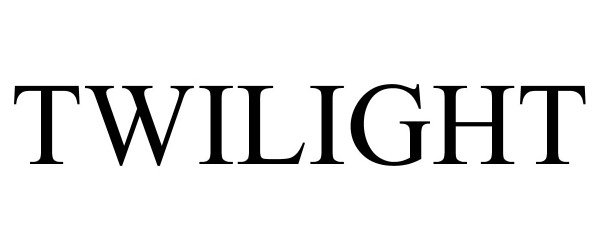 TWILIGHT 90689038 not registered Live/Pending |
Campbell, Hensley 2021-05-04 |
 TWILIGHT 90492151 not registered Live/Pending |
Lanshing Pty Ltd 2021-01-27 |
 TWILIGHT 88649490 not registered Live/Pending |
Heartland Recreational Vehicles, LLC 2019-10-10 |
 TWILIGHT 88292683 not registered Live/Pending |
Ruca 1982, Inc. 2019-02-07 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.