DIGIT WIDGET
Pin, Fixation, Threaded
HAND BIOMECHANICS LAB, INC.
The following data is part of a premarket notification filed by Hand Biomechanics Lab, Inc. with the FDA for Digit Widget.
Pre-market Notification Details
| Device ID | K992970 |
| 510k Number | K992970 |
| Device Name: | DIGIT WIDGET |
| Classification | Pin, Fixation, Threaded |
| Applicant | HAND BIOMECHANICS LAB, INC. 77 SCRIPPS DR., SUITE 104 Sacramento, CA 95825 |
| Contact | John M Agee |
| Correspondent | John M Agee HAND BIOMECHANICS LAB, INC. 77 SCRIPPS DR., SUITE 104 Sacramento, CA 95825 |
| Product Code | JDW |
| CFR Regulation Number | 888.3040 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 1999-09-03 |
| Decision Date | 1999-11-08 |
| Summary: | summary |
NIH GUDID Devices
| Device Identifier | submissionNumber | Supplement |
|---|---|---|
| 00861994000212 | K992970 | 000 |
Trademark Results [DIGIT WIDGET]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
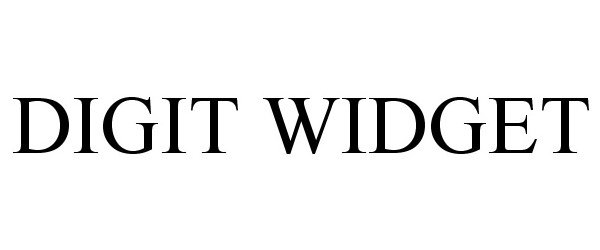 DIGIT WIDGET 86187416 4636036 Live/Registered |
John M. Agee 2014-02-07 |
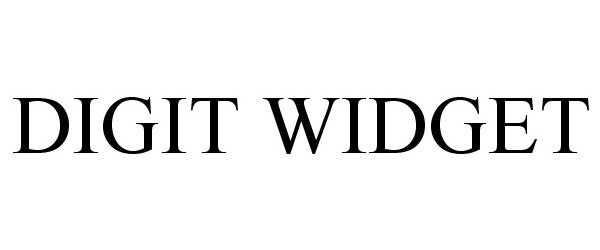 DIGIT WIDGET 85376032 4138043 Dead/Cancelled |
Siek, Jason Louis 2011-07-20 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.