BIOPACK
Lamp, Infrared, Therapeutic Heating
BIOSCAN, INC.
The following data is part of a premarket notification filed by Bioscan, Inc. with the FDA for Biopack.
Pre-market Notification Details
| Device ID | K993685 |
| 510k Number | K993685 |
| Device Name: | BIOPACK |
| Classification | Lamp, Infrared, Therapeutic Heating |
| Applicant | BIOSCAN, INC. 6 WALDEN RD. Corrales, NM 87048 |
| Contact | Butch Smith |
| Correspondent | Butch Smith BIOSCAN, INC. 6 WALDEN RD. Corrales, NM 87048 |
| Product Code | ILY |
| CFR Regulation Number | 890.5500 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 1999-11-01 |
| Decision Date | 2000-07-18 |
Trademark Results [BIOPACK]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
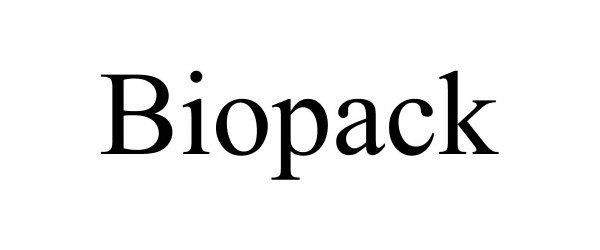 BIOPACK 90236831 not registered Live/Pending |
Armatura Global Solutions 2020-10-06 |
 BIOPACK 87377829 not registered Dead/Abandoned |
NWT Enterprises Ltd. 2017-03-20 |
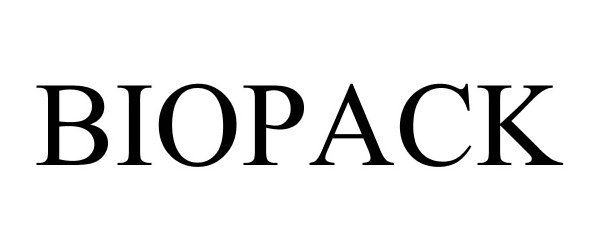 BIOPACK 86713876 not registered Dead/Abandoned |
Valencell, Inc. 2015-08-04 |
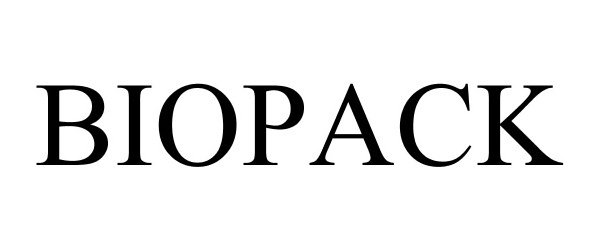 BIOPACK 85223247 not registered Dead/Abandoned |
Reveal Therapies, LLC 2011-01-21 |
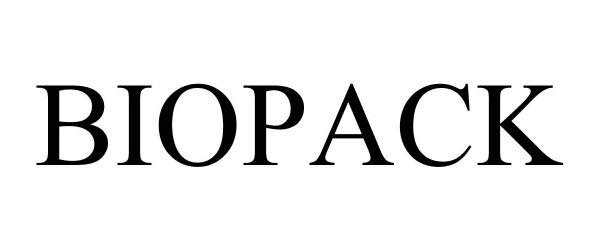 BIOPACK 78681982 not registered Dead/Abandoned |
Food Packaging Solutions, Inc. 2005-07-29 |
 BIOPACK 75085334 2166794 Dead/Cancelled |
BioScan, Inc. 1996-04-08 |
 BIOPACK 73223261 1399153 Dead/Cancelled |
BIOTEST-SERUM-INSTITUT GMBH 1979-07-13 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.