NYLON
Suture, Nonabsorbable, Synthetic, Polyamide
TRADING CONSULTANTS AND DISTRIBUTORS INTL., INC.
The following data is part of a premarket notification filed by Trading Consultants And Distributors Intl., Inc. with the FDA for Nylon.
Pre-market Notification Details
| Device ID | K993998 |
| 510k Number | K993998 |
| Device Name: | NYLON |
| Classification | Suture, Nonabsorbable, Synthetic, Polyamide |
| Applicant | TRADING CONSULTANTS AND DISTRIBUTORS INTL., INC. 157 WINDDANCE DR. Chicago, IL 60046 |
| Contact | Main M Ghazal |
| Correspondent | Main M Ghazal TRADING CONSULTANTS AND DISTRIBUTORS INTL., INC. 157 WINDDANCE DR. Chicago, IL 60046 |
| Product Code | GAR |
| CFR Regulation Number | 878.5020 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 1999-11-24 |
| Decision Date | 2000-02-04 |
| Summary: | summary |
Trademark Results [NYLON]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
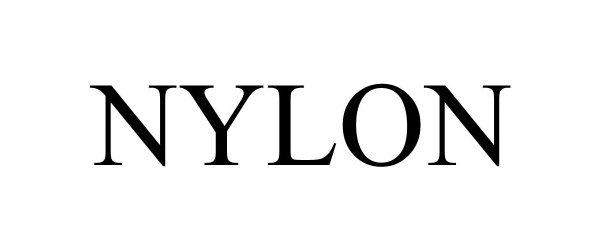 NYLON 98094638 not registered Live/Pending |
BDG Media, Inc. 2023-07-20 |
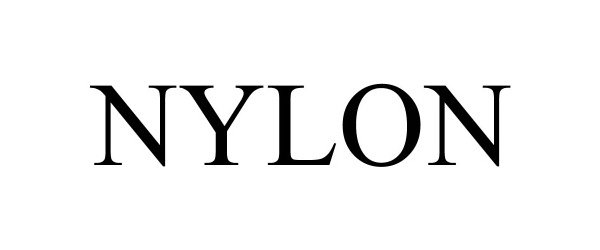 NYLON 90453767 not registered Live/Pending |
BDG Media, Inc. 2021-01-07 |
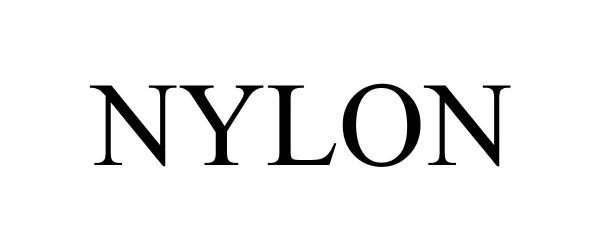 NYLON 85885426 4668157 Live/Registered |
Nylon Media, Inc. 2013-03-25 |
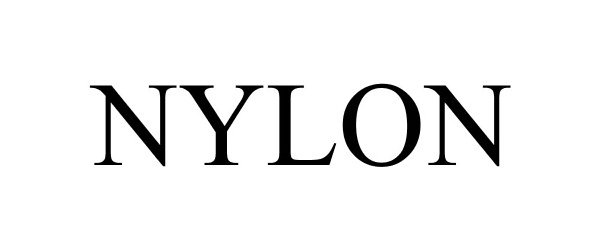 NYLON 85885418 not registered Dead/Abandoned |
NYLON MEDIA, INC. 2013-03-25 |
 NYLON 85885412 not registered Dead/Abandoned |
NYLON MEDIA, INC. 2013-03-25 |
 NYLON 85885407 not registered Dead/Abandoned |
NYLON MEDIA, INC. 2013-03-25 |
 NYLON 85885403 4672358 Live/Registered |
NYLON MEDIA, INC. 2013-03-25 |
 NYLON 85885394 not registered Dead/Abandoned |
NYLON MEDIA, INC. 2013-03-25 |
 NYLON 79052496 3661040 Dead/Cancelled |
Nylon Studios Pty Limited 2008-03-04 |
 NYLON 77405209 3601225 Live/Registered |
BDG Media, Inc. 2008-02-25 |
 NYLON 76367518 not registered Dead/Abandoned |
Brunetti, Mary J. 2002-02-06 |
 NYLON 75942735 not registered Dead/Abandoned |
Nylon, L.L.C. 2000-03-07 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.