OXICONAZOLE NITRATE cream
Oxiconazole Nitrate by
Drug Labeling and Warnings
Oxiconazole Nitrate by is a Prescription medication manufactured, distributed, or labeled by Taro Pharmaceuticals U.S.A., Inc., Taro Pharmaceuticals Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Oxiconazole nitrate cream, 1% contains the antifungal active compound oxiconazole nitrate. This formulation is for topical dermatologic use only.
Chemically, oxiconazole nitrate is 2',4'-dichloro-2-imidazol-1-ylacetophenone (Z)-[0-(2,4-dichlorobenzyl)oxime], mononitrate. The compound has the molecular formula C18H13ON3CI4HNO3, a molecular weight of 492.15, and the following structural formula:
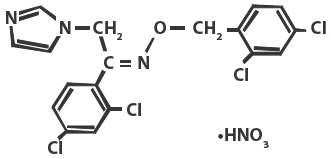
Oxiconazole nitrate is a nearly white crystalline powder, soluble in methanol; sparingly soluble in ethanol, chloroform, and acetone; and very slightly soluble in water.
Oxiconazole nitrate cream, 1% contains 10 mg of oxiconazole per gram of cream in a white to off-white cream base of cetyl alcohol, polysorbate 60, propylene glycol, purified water, stearyl alcohol, white petrolatum, and benzoic acid 0.2% as a preservative.
-
CLINICAL PHARMACOLOGY
Pharmacokinetics
The penetration of oxiconazole nitrate into different layers of the skin was assessed using an in vitro permeation technique with human skin. Five hours after application of 2.5 mg/cm2 of oxiconazole nitrate cream onto human skin, the concentration of oxiconazole nitrate was demonstrated to be 16.2 μmol in the epidermis, 3.64 μmol in the upper corium, and 1.29 μmol in the deeper corium. Systemic absorption of oxiconazole nitrate is low. Using radiolabeled drug, less than 0.3% of the applied dose of oxiconazole nitrate was recovered in the urine of volunteer subjects up to 5 days after application of the cream formulation. Neither in vitro nor in vivo studies have been conducted to establish relative activity between the lotion and cream formulations.
Microbiology
Oxiconazole nitrate is an imidazole derivative whose antifungal activity is derived primarily from the inhibition of ergosterol biosynthesis, which is critical for cellular membrane integrity. It has in vitro activity against a wide range of pathogenic fungi.
Oxiconazole has been shown to be active against most strains of the following organisms both in vitro and in clinical infections at indicated body sites (see INDICATIONS AND USAGE):
- Epidermophyton floccosum
- Trichophyton mentagrophytes
- Trichophyton rubrum
- Malassezia furfur
The following in vitro data are available; however, their clinical significance is unknown. Oxiconazole exhibits satisfactory in vitro minimum inhibitory concentrations (MICs) against most strains of the following organisms; however, the safety and efficacy of oxiconazole in treating clinical infections due to these organisms have not been established in adequate and well-controlled clinical trials:
- Candida albicans
- Microsporum audouini
- Microsporum canis
- Microsporum gypseum
- Trichophyton tonsurans
- Trichophyton violaceum
-
INDICATIONS AND USAGE
Oxiconazole nitrate cream is indicated for the topical treatment of the following dermal infections: tinea pedis, tinea cruris, and tinea corporis due to Trichophyton rubrum, Trichophyton mentagrophytes, or Epidermophyton floccosum. Oxiconazole nitrate cream is indicated for the topical treatment of tinea (pityriasis) versicolor due to Malassezia furfur (see DOSAGE AND ADMINISTRATION and CLINICAL STUDIES).
Oxiconazole nitrate cream may be used in pediatric patients for tinea corporis, tinea cruris, tinea pedis, and tinea (pityriasis) versicolor; however, these indications for which oxiconazole nitrate cream has been shown to be effective rarely occur in children below the age of 12.
- CONTRAINDICATIONS
- WARNINGS
-
PRECAUTIONS
General
Oxiconazole nitrate cream is for external dermal use only. Avoid introduction of oxiconazole nitrate cream into the eyes or vagina. If a reaction suggesting sensitivity or chemical irritation should occur with the use of oxiconazole nitrate cream, treatment should be discontinued and appropriate therapy instituted. If signs of epidermal irritation should occur, the drug should be discontinued.
Information for Patients
The patient should be instructed to:
- Use oxiconazole nitrate cream as directed by the physician. The hands should be washed after applying the medication to the affected area(s). Avoid contact with the eyes, nose, mouth, and other mucous membranes. Oxiconazole nitrate cream is for external use only.
- Use the medication for the full treatment time recommended by the physician, even though symptoms may have improved. Notify the physician if there is no improvement after 2 to 4 weeks, or sooner if the condition worsens (see below).
- Inform the physician if the area of application shows signs of increased irritation, itching, burning, blistering, swelling, or oozing.
- Avoid the use of occlusive dressings unless otherwise directed by the physician.
- Do not use this medication for any disorder other than that for which it was prescribed.
Drug Interactions
Potential drug interactions between oxiconazole nitrate cream and other drugs have not been systematically evaluated.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Although no long-term studies in animals have been performed to evaluate carcinogenic potential, no evidence of mutagenic effect was found in 2 mutation assays (Ames test and Chinese hamster V79 in vitro cell mutation assay) or in 2 cytogenetic assays (human peripheral blood lymphocyte in vitro chromosome aberration assay and in vivo micronucleus assay in mice).
Reproductive studies revealed no impairment of fertility in rats at oral doses of 3 mg/kg/day in females (1 time the human dose based on mg/m2) and 15 mg/kg/day in males (4 times the human dose based on mg/m2). However, at doses above this level, the following effects were observed: a reduction in the fertility parameters of males and females, a reduction in the number of sperm in vaginal smears, extended estrous cycle, and a decrease in mating frequency.
Pregnancy
Teratogenic Effects
Pregnancy Category B
Reproduction studies have been performed in rabbits, rats, and mice at oral doses up to 100, 150, and 200 mg/kg/day (57, 40, and 27 times the human dose based on mg/m2), respectively, and revealed no evidence of harm to the fetus due to oxiconazole nitrate. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Nursing Mothers
Because oxiconazole is excreted in human milk, caution should be exercised when the drug is administered to a nursing woman.
Pediatric Use
Oxiconazole nitrate cream may be used in pediatric patients for tinea corporis, tinea cruris, tinea pedis, and tinea (pityriasis) versicolor; however, these indications for which oxiconazole nitrate cream has been shown to be effective rarely occur in children below the age of 12.
Geriatric Use
A limited number of patients at or above 60 years of age (n ~ 396) have been treated with oxiconazole nitrate cream in US and non-US clinical trials, and a limited number (n = 43) have been treated with oxiconazole nitrate lotion in US clinical trials. The number of patients is too small to permit separate analysis of efficacy and safety. No adverse events were reported with oxiconazole nitrate lotion in geriatric patients, and the adverse reactions reported with oxiconazole nitrate cream in this population were similar to those reported by younger patients. Based on available data, no adjustment of dosage of oxiconazole nitrate cream in geriatric patients is warranted.
-
ADVERSE REACTIONS
During clinical trials, of 955 patients treated with oxiconazole nitrate cream, 1%, 41 (4.3%) reported adverse reactions thought to be related to drug therapy. These reactions included pruritus (1.6%); burning (1.4%); irritation and allergic contact dermatitis (0.4% each); folliculitis (0.3%); erythema (0.2%); and papules, fissure, maceration, rash, stinging, and nodules (0.1% each).
In a controlled, multicenter clinical trial of 269 patients treated with oxiconazole nitrate lotion, 1%, 7 (2.6%) reported adverse reactions thought to be related to drug therapy. These reactions included burning and stinging (0.7% each) and pruritus, scaling, tingling, pain, and dyshidrotic eczema (0.4% each).
-
OVERDOSAGE
When 5% oxiconazole cream (5 times the concentration of the marketed product) was applied at a rate of 1 g/kg to approximately 10% of body surface area of a group of 40 male and female rats for 35 days, 3 deaths and severe dermal inflammation were reported. No overdoses in humans have been reported with use of oxiconazole nitrate cream or lotion.
-
DOSAGE AND ADMINISTRATION
Oxiconazole nitrate cream should be applied to affected and immediately surrounding areas once to twice daily in patients with tinea pedis, tinea corporis, or tinea cruris. Oxiconazole nitrate cream should be applied once daily in the treatment of tinea (pityriasis) versicolor. Tinea corporis, tinea cruris, and tinea (pityriasis) versicolor should be treated for 2 weeks and tinea pedis for 1 month to reduce the possibility of recurrence. If a patient shows no clinical improvement after the treatment period, the diagnosis should be reviewed.
Note: Tinea (pityriasis) versicolor may give rise to hyperpigmented or hypopigmented patches on the trunk that may extend to the neck, arms, and upper thighs. Treatment of the infection may not immediately result in restoration of pigment to the affected sites. Normalization of pigment following successful therapy is variable and may take months, depending on individual skin type and incidental sun exposure. Although tinea (pityriasis) versicolor is not contagious, it may recur because the organism that causes the disease is part of the normal skin flora.
-
CLINICAL STUDIES
The following definitions were applied to the clinical and microbiological outcomes in patients enrolled in the clinical trials that form the basis for the approvals of Oxiconazole nitrate lotion and Oxiconazole nitrate cream.
Definitions
- Mycological Cure: No evidence (culture and KOH preparation) of the baseline (original) pathogen in a specimen from the affected area taken at the 2-week post-treatment visit (for tinea [pityriasis] versicolor, mycological cure was limited to KOH only).
- Treatment Success: Both a global evaluation of ≥90% clinical improvement and a microbiologic eradication (see above) at the 2-week post-treatment visit.
Tinea Pedis
THERE ARE NO HEAD-TO-HEAD COMPARISON TRIALS OF THE OXICONAZOLE NITRATE CREAM AND LOTION FORMULATIONS IN THE TREATMENT OF TINEA PEDIS.
Lotion Formulation
The clinical trial for the lotion formulation line extension involved 332 evaluable patients with clinically and microbiologically established tinea pedis. Of these evaluable patients, 64% were diagnosed with hyperkeratotic plantar tinea pedis and 28% with interdigital tinea pedis. Seventy-seven percent (77%) had disease secondary to infection with Trichophyton rubrum, 18% had disease secondary to infection with Trichophyton mentagrophytes, and 4% had disease secondary to infection with Epidermophyton floccosum.
The results of this clinical trial at the 2-week post-treatment follow-up visit are shown in the following table:
Patient Outcome Oxiconazole nitrate lotion Vehicle b.i.d q.d. Myocological cure 67% 64% 28% Treatment success 41% 34% 10% In this study, the improvement and cure rates of the b.i.d.- and q.d.-treated groups did not differ significantly (95% confidence interval) from each other but were statistically (95% confidence interval) superior to the vehicle-treated group.
Cream Formulation
The two pivotal trials for the cream formulation involved 281 evaluable patients (total from both trials) with clinically and microbiologically established tinea pedis.
The combined results of these two clinical trials at the 2-week post-treatment follow-up visit are shown in the following table:
Patient Outcome Oxiconazole nitrate cream Vehicle b.i.d q.d. Myocological cure 77% 79% 33% Treatment success 52% 43% 14% All the improvement and cure rates of the b.i.d.- and q.d.- treated groups did not differ significantly (95% confidence interval) from each other but were statistically (95% confidence interval) superior to the vehicle-treated group.
In addition, pediatric data (95 children ages 10 and under) available with the cream formulation indicate that it is safe and effective for use in children when used as directed. Adverse events were reported in 2 children; 1 child was reported to have reddening of the skin and 1 child was reported to have eczema-like skin alterations.
Tinea (pityriasis) Versicolor
Two pivotal clinical trials of oxiconazole nitrate cream in tinea (pityriasis) versicolor involved 219 evaluable patients in the q day oxiconazole nitrate and vehicle arms of the trial with clinical and mycological evidence of tinea (pityriasis) versicolor. Patients were treated for 2 weeks with oxiconazole nitrate cream once daily, or with cream vehicle. The combined results of these clinical trials at the 2-week post-treatment follow-up visit are shown in the following table. These results are based on 207 patients (110 in the oxiconazole nitrate group and 97 in the vehicle group) with efficacy evaluations at this visit.
Patient Outcome Oxiconazole nitrate cream Vehicle q.d. Myocological cure 88% 67% Treatment success 83% 62% Only once a day was shown in both studies to be statistically superior to vehicle for all efficacy parameters at 2 weeks and follow-up.
-
HOW SUPPLIED
Oxiconazole Nitrate Cream, 1% is supplied in:
15 g tubes - (NDC: 51672-1359-1)
30 g tubes - (NDC: 51672-1359-2)
60 g tubes - (NDC: 51672-1359-3)
90 g tubes - (NDC: 51672-1359-8) - SPL UNCLASSIFIED SECTION
-
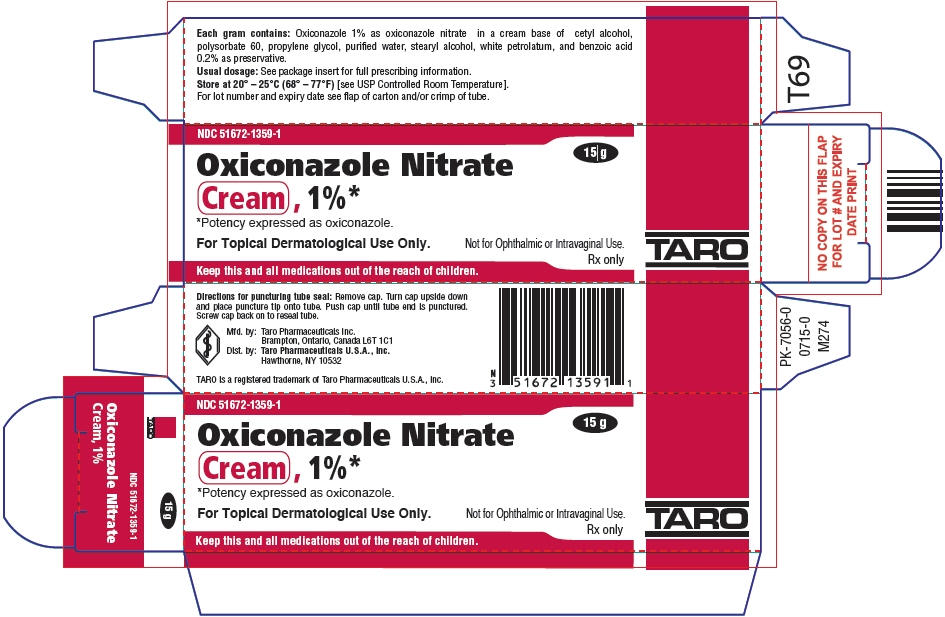
PRINCIPAL DISPLAY PANEL - 15 g Tube Carton
NDC: 51672-1359-1
15 g
Oxiconazole Nitrate
Cream , 1%*
*Potency expressed as oxiconazole.For Topical Dermatological Use Only.
Not for Ophthalmic or Intravaginal Use.Rx only
Keep this and all medications out of the reach of children.
TARO
-
INGREDIENTS AND APPEARANCE
OXICONAZOLE NITRATE
oxiconazole nitrate creamProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 51672-1359 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Oxiconazole Nitrate (UNII: RQ8UL4C17S) (Oxiconazole - UNII:C668Q9I33J) Oxiconazole 10 mg in 1 g Inactive Ingredients Ingredient Name Strength cetyl alcohol (UNII: 936JST6JCN) polysorbate 60 (UNII: CAL22UVI4M) propylene glycol (UNII: 6DC9Q167V3) water (UNII: 059QF0KO0R) stearyl alcohol (UNII: 2KR89I4H1Y) petrolatum (UNII: 4T6H12BN9U) benzoic acid (UNII: 8SKN0B0MIM) Product Characteristics Color WHITE (white to off-white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 51672-1359-1 1 in 1 CARTON 03/07/2016 1 15 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC: 51672-1359-2 1 in 1 CARTON 03/07/2016 2 30 g in 1 TUBE; Type 0: Not a Combination Product 3 NDC: 51672-1359-3 1 in 1 CARTON 03/07/2016 3 60 g in 1 TUBE; Type 0: Not a Combination Product 4 NDC: 51672-1359-8 1 in 1 CARTON 03/07/2016 4 90 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA205076 03/07/2016 Labeler - Taro Pharmaceuticals U.S.A., Inc. (145186370) Establishment Name Address ID/FEI Business Operations Taro Pharmaceuticals Inc. 206263295 MANUFACTURE(51672-1359) , API MANUFACTURE(51672-1359)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.